EPA Methods List with Links
US EPA Method 5E - Determination Of Particulate Matter Emissions From The Wool Fiberglass Insulation Manufacturing Industry
NOTE: This method does not include all of the specifications (e.g., equipment and supplies) and procedures (e.g., sampling and analytical) essential to its performance. Some material is incorporated by reference from other methods in this part. Therefore, to obtain reliable results, persons using this method should have a thorough knowledge of at least the following additional test methods: Method 1, Method 2, Method 3, and Method 5.
Content [ show/hide ].1.0 Scope and Applications.
1.1 Analyte.
Particulate matter (PM). No CAS number assigned.
1.2 Applicability.
This method is applicable for the determination of PM emissions from wool fiberglass insulation manufacturing sources.
2.0 Summary of Method.
Particulate matter is withdrawn isokinetically from the source and is collected either on a glass fiber filter maintained at a temperature in the range of 120 ± 14 C (248 ± 25 F) and in impingers in solutions of 0.1 N sodium hydroxide (NaOH). The filtered particulate mass, which includes any material that condenses at or above the filtration temperature, is determined gravimetrically after the removal of uncombined water. The condensed PM collected in the impinger solutions is determined as total organic carbon (TOC) using a nondispersive infrared type of analyzer. The sum of the filtered PM mass and the condensed PM is reported as the total PM mass.
3.0 Definitions. [Reserved]
4.0 Interferences. [Reserved]
5.0 Safety.
5.1 Disclaimer.
This method may involve hazardous materials, operations, and equipment. This test method may not address all of the safety problems associated with its use. It is the responsibility of the user of this test method to establish appropriate safety and health practices and to determine the applicability of regulatory limitations prior to performing this test method.
5.2 Corrosive Reagents.
The following reagents are hazardous. Personal protective equipment and safe procedures are useful in preventing chemical splashes. If contact occurs, immediately flush with copious amounts of water at least 15 minutes. Remove clothing under shower and decontaminate. Treat residual chemical burn as thermal burn.
5.2.1 Hydrochloric Acid (HCl). Highly toxic.
Vapors are highly irritating to eyes, skin, nose, and lungs, causing severe damage. May cause bronchitis, pneumonia, or edema of lungs. Exposure to concentrations of 0.13 to 0.2 percent in air can be lethal in minutes. Will react with metals, producing hydrogen.
5.2.2 Sodium Hydroxide (NaOH).
Causes severe damage to eye tissues and to skin. Inhalation causes irritation to nose, throat, and lungs. Reacts exothermically with limited amounts of water.
6.0 Equipment and Supplies.
6.1 Sample Collection.
Same as Method 5, Section 6.1, with the exception of the following:
6.1.1 Probe liner.
Same as described in Section 6.1.1.2 of Method 5 except use only borosilicate or quartz glass liners.
6.1.2 filter Holder.
Same as described in Section 6.1.1.5 of Method 5 with the addition of a leak-tight connection in the rear half of the filter holder designed for insertion of a tenperature sensor used for measuring the sample gas exit temperature.
6.2 sample recovery.
Same as Method 5, Section 6.2, except three wash bottles are needed instead of two and only glass storage bottles and funnels may be used.
6.3 Sample Analysis.
Same as Method 5, Section 6.3, with the additional equipment for TOC analysis as described below:
6.3.1 Sample Blender or Homogenizer.
Waring type or ultrasonic.
6.3.2 Magnetic Stirrer.
6.3.3 Hypodermic Syringe.
0- to 100-l capacity.
6.3.4 Total Organic Carbon Analyzer.
Rosemount Model 2100A analyzer or equivalent and a recorder.
6.3.5 Beaker.
30-ml.
6.3.6 Water Bath.
temperature controlled.
6.3.7 Volumetric Flasks.
1000-ml and 500-ml.
7.0 Reagents and Standards.
Unless otherwise indicated, it is intended that all reagents conform to the specifications established by the Committee on Analytical Reagents of the American Chemical Society, where such specifications are available; otherwise, use the best available grade.
7.1 Sample Collection.
Same as Method 5, Section 7.1, with the addition of 0.1 N NaOH (Dissolve 4 g of NaOH in water and dilute to 1 liter).
7.2 sample recovery.
Same as Method 5, Section 7.2, with the addition of the following:
7.2.1 Water.
Deionized distilled to conform to ASTM Specification D 1193-77 or 91 Type 3 (incorporated by reference - see 60.17). The potassium permanganate (KMnO<sub>4</sub>) test for oxidizable organic matter may be omitted when high concentrations of organic matter are not expected to be present.
7.2.2 Sodium Hydroxide.
Same as described in Section 7.1.
7.3 Sample Analysis.
Same as Method 5, Section 7.3, with the addition of the following:
7.3.1 Carbon Dioxide-Free Water.
Distilled or deionized water that has been freshly boiled for 15 minutes and cooled to room temperature while preventing exposure to ambient air by using a cover vented with an Ascarite tube.
7.3.2 Hydrochloric Acid.
HCl, concentrated, with a dropper.
7.3.3 Organic Carbon Stock Solution.
Dissolve 2.1254 g of dried potassium biphthalate (HOOCC6H4COOK) in CO2-free water, and dilute to 1 liter in a volumetric flask. This solution contains 1000 mg/L organic carbon.
7.3.4 Inorganic Carbon Stock Solution.
Dissolve 4.404 g anhydrous sodium carbonate (Na2CO3) in about 500 ml of CO2-free water in a 1-liter volumetric flask. Add 3.497 g anhydrous sodium bicarbonate (NaHCO3) to the flask, and dilute to 1 liter with CO2-free water. This solution contains 1000 mg/L inorganic carbon.
7.3.5 Oxygen Gas.
CO2-free.
8.0 Sample Collection, Preservation, Storage, and Transport.
8.1 Pretest Preparation and Preliminary Determinations.
Same as Method 5, Sections 8.1 and 8.2, respectively.
8.2 Preparation of Sampling train.
Same as Method 5, Section 8.3, except that 0.1 M NaOH is used in place of water in the impingers. The volumes of the solutions are the same as in Method 5.
8.3 Leak-Check Procedures, Sampling train Operation, Calculation of Percent Isokinetic.
Same as Method 5, Sections 8.4 through 8.6, respectively.
8.4 sample recovery.
Same as Method 5, Sections 8.7.1 through 8.7.4, with the addition of the following:
8.4.1 Save portions of the water, acetone, and 0.1 N NaOH used for cleanup as blanks. Take 200 ml of each liquid directly from the wash bottles being used, and place in glass sample containers labeled "water blank," "acetone blank," and "NaOH blank," respectively.
8.4.2 Inspect the train prior to and during disassembly, and note any abnormal conditions. Treat the samples as follows:
8.4.2.1 Container No. 1.
Same as Method 5, Section 8.7.6.1.
8.4.2.2 Container No. 2.
Use water to rinse the sample probe nozzle, Probe, and front half of the filter holder three times in the manner described in Section 8.7.6.2 of Method 5 except that no brushing is done. Put all the water wash in one container, seal, and label.
8.4.2.3 Container No. 3.
Rinse and brush the sample probe nozzle, Probe, and front half of the filter holder with acetone as described for Container No. 2 in Section 8.7.6.2 of Method 5.
8.4.2.4 Container No. 4.
Place the contents of the silica gel impinger in its original container as described for Container No. 3 in Section 8.7.6.3 of Method 5.
8.4.2.5 Container No. 5.
Measure the liquid in the first three impingers and record the volume or weight as described for the impinger Water in Section 8.7.6.4 of Method 5. Do not discard this liquid, but place it in a sample container using a glass funnel to aid in the transfer from the impingers or graduated cylinder (if used) to the sample container. Rinse each impinger thoroughly with 0.1 N NaOH three times, as well as the graduated cylinder (if used) and the funnel, and put these rinsings in the same sample container. Seal the container and label to clearly identify its contents.
8.5 Sample Transport.
Whenever possible, containers should be shipped in such a way that they remain upright at all times.
9.0 Quality Control.
9.1 Miscellaneous Quality Control Measures.
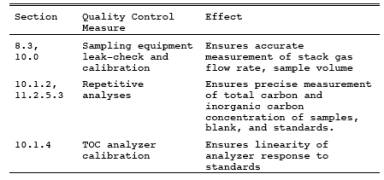
9.2 Volume metering System Checks.
Same as Method 5, Section 9.2.
10.0 Calibration and Standardization.
Same as Method 5, Section 10.0, with the addition of the following procedures for calibrating the total organic carbon analyzer:
10.1 Preparation of Organic Carbon Standard Curve.
10.1.1 Add 10 ml, 20 ml, 30 ml, 40 ml, and 50 ml of the organic carbon stock solution to a series of five 1000-ml volumetric flasks. Add 30 ml, 40 ml, and 50 ml of the same solution to a series of three 500-ml volumetric flasks. Dilute the contents of each flask to the mark using CO2-free water. These flasks contain 10, 20, 30, 40, 50, 60, 80, and 100 mg/L organic carbon, respectively.
10.1.2 Use a hypodermic syringe to withdraw a 20- to 50-l aliquot from the 10 mg/L standard solution and inject it into the total carbon port of the analyzer. Measure the peak height. Repeat the injections until three consecutive peaks are obtained within 10 percent of their arithmetic mean. Repeat this procedure for the remaining organic carbon standard solutions.
10.1.3 Calculate the corrected peak height for each standard by deducting the blank correction (see Section 11.2.5.3) as follows:
Corrected Peak Height = A - B Eq. 5E-1
where:
| A | = | Peak height of standard or sample, mm or other appropriate unit. |
| B | = | Peak height of blank, mm or other appropriate unit. |
10.1.4 Prepare a linear regression plot of the arithmetic mean of the three consecutive peak heights obtained for each standard solution against the concentration of that solution. Calculate the calibration factor as the inverse of the slope of this curve. If the product of the arithmetic mean peak height for any standard solution and the calibration factor differs from the actual concentration by more than 5 percent, remake and reanalyze that standard.
10.2 Preparation of Inorganic Carbon Standard Curve.
Repeat the procedures outlined in Sections 10.1.1 through 10.1.4, substituting the inorganic carbon stock solution for the organic carbon stock solution, and the inorganic carbon port of the analyzer for the total carbon port.
11.0 Analytical Procedure.
11.1 Record the data required on a sheet such as the one shown in Figure 5-6 of Method 5.
11.2 Handle each sample container as follows:
11.2.1 Container No. 1.
Same as Method 5, Section 11.2.1, except that the filters must be dried at 20 ± 6 C (68 ± 10 F) and ambient pressure.
11.2.2 Containers No. 2 and No. 3.
Same as Method 5, Section 11.2.2, except that evaporation of the samples must be at 20 ± 6 C (68 ± 10 F) and ambient pressure.
11.2.3 Container No. 4.
Same as Method 5, Section 11.2.3.
11.2.4 "Water Blank" and "Acetone Blank" Containers.
Determine the water and acetone blank values following the procedures for the "Acetone Blank" container in Section 11.2.4 of Method 5. Evaporate the samples at ambient temperature [20 ± 6 C (68 ± 10 F)] and pressure.
11.2.5 Container No. 5.
For the determination of total organic carbon, perform two analyses on successive identical samples, i.e., total carbon and inorganic carbon. The desired quantity is the difference between the two values obtained. Both analyses are based on conversion of sample carbon into carbon dioxide for measurement by a nondispersive infrared analyzer. Results of analyses register as peaks on a strip chart recorder.
11.2.5.1 The principal differences between the operating parameters for the two channels involve the combustion tube packing material and temperature. In the total carbon channel, a high temperature [950 C (1740 F)] furnace heats a Hastelloy combustion tube packed with cobalt oxide-impregnated asbestos fiber. The oxygen in the carrier gas, the elevated temperature, and the catalytic effect of the packing result in oxidation of both organic and inorganic carbonaceous material to CO2 and steam. In the inorganic carbon channel, a low temperature [150 C (300 F)] furnace heats a glass tube containing quartz chips wetted with 85 percent phosphoric acid. The acid liberates CO2 and steam from inorganic carbonates. The operating temperature is below that required to oxidize organic matter. Follow the manufacturer's instructions for assembly, testing, calibration, and operation of the analyzer.
11.2.5.2 As samples collected in 0.1 N NaOH often contain a high measure of inorganic carbon that inhibits repeatable determinations of TOC, sample pretreatment is necessary. Measure and record the liquid volume of each sample (or impinger contents). If the sample contains solids or immiscible liquid matter, homogenize the sample with a blender or ultrasonics until satisfactory repeatability is obtained. Transfer a representative portion of 10 to 15 ml to a 30-ml beaker, and acidify with about 2 drops of concentrated HCl to a pH of 2 or less. Warm the acidified sample at 50 C (120 F) in a water bath for 15 minutes.
11.2.5.3 While stirring the sample with a magnetic stirrer, use a hypodermic syringe to withdraw a 20- to 50-l aliquot from the beaker. Analyze the sample for total carbon and calculate its corrected mean peak height according to the procedures outlined in Sections 10.1.2 and 10.1.3. Similarly analyze an aliquot of the sample for inorganic carbon. Repeat the analyses for all the samples and for the 0.1 N NaOH blank.
11.2.5.4 Ascertain the total carbon and inorganic carbon concentrations (CTC and CIC, respectively) of each sample and blank by comparing the corrected mean peak heights for each sample and blank to the appropriate standard curve.
NOTE: If samples must be diluted for analysis, apply an appropriate dilution factor.
12.0 Data Analysis and Calculations.
Same as Method 5, Section 12.0, with the addition of the following:
12.1 Nomenclature.
| Cc | = | Concentration of condensed particulate matter in stack gas, gas dry basis, corrected to standard conditions, g/dscm (gr/dscf). |
| CIC | = | Concentration of condensed TOC in the liquid sample, from Section 11.2.5, mg/L. |
| Ct | = | Total particulate concentration, dry basis, corrected to standard conditions, g/dscm (gr/dscf). |
| CTC | = | Concentration of condensed TOC in the liquid sample, from Section 11.2.5, mg/L. |
| CTOC | = | Concentration of condensed TOC in the liquid sample, mg/L. |
| mTOC | = | Mass of condensed TOC collected in the impingers, mg. |
| Vm(std) | = | Volume of gas sample measured by the console meter, corrected to standard conditions, from Section 12.3 of Method 5, dscm (dscf). |
| Vs | = | Total volume of liquid sample, ml. |
12.2 Concentration of Condensed TOC in Liquid Sample.

12.3 Mass of Condensed TOC Collected.

where:
0.001 = Liters per milliliter.
12.4 Concentration of Condensed Particulate Material.

where:
| K4 | = | 0.001 g/mg for metric units. |
| = | 0.0154 gr/mg for English units. |
12.5 Total Particulate Concentration.

13.0 Method Performance. [Reserved]
14.0 Pollution Prevention. [Reserved]
15.0 Waste Management. [Reserved]
16.0 References.
Same as Section 17.0 of Method 5, with the addition of the following:
1. American Public Health Association, American Water Works Association, Water Pollution Control Federation. Standard Methods for the Examination of Water and Wastewater. Fifteenth Edition. Washington, D.C. 1980.
17.0 Tables, Diagrams, flowcharts, and Validation Data. [Reserved]
- Analytical
- Ion Chromatography
- Gas Chromatography
- Gravimetrics
- Ash Resistivity
- Inks/Coatings
- Scrubber Stoichiometry
- Titrations
- Mercury Sorbent Trap
- Engineering
- Express Products
- Rental Instruments
- MET80 Mercury Monitor
- Continuous Emission Monitors
- Gas Sampling Equipment
- Mobile Power Supply
Our Resources
- Technical Resources

 Express
Express FTIR
FTIR Mercury
Mercury Emission Sampling Equipment
Emission Sampling Equipment Instrument Rental
Instrument Rental