EPA Methods List with Links
US EPA Method 23 - Determination of Polychlorinated Dibenzo-p-dioxins and Polychlorinated Dibenzofurans from Municipal Waste Combustors
Necessary equipment for performing Method 23
Content [ show/hide ].1. APPLICABILITY AND PRINCIPLE
1.1 Applicability.
This method is applicable to the determination of emissions of polychlorinated dibenzo-p-dioxins (PCDD's) and polychlorinated dibenzofurans (PCDF's) from stationary sources.
1.2 Principle.
A sample is withdrawn isokinetically from the gas stream and collected in the sample Probe, on a glass fiber filter, and on a packed column of adsorbent material. The sample cannot be separated into a particle and vapor fraction. The PCDD's and PCDF's are extracted from the sample, separated by high resolution gas chromatography (HRGC), and measured by high resolution mass spectrometry (HRMS).
2. APPARATUS
2.1 Sampling.
A schematic of the sampling train is shown in Figure 23-1. Sealing greases may not be used in assembling the train. The train is identical to that described in Section 2.1 of Method 5 of this appendix with the following additions:
2.1.1 probe nozzle. The probe nozzle shall be made of nickel, nickel-plated stainless steel, quartz, or borosilicate glass.
2.1.2 Sample Transfer Lines. The sample transfer lines, if needed, shall be heat traced, heavy walled TFE (1/2 in. OD with 1/8 in. wall) with connecting fittings that are capable of forming leak free, vacuum-tight connections without using sealing greases. The line shall be as short as possible and must be maintained at 120C.
2.1.3 Filter Support. Teflon or Teflon-coated wire.
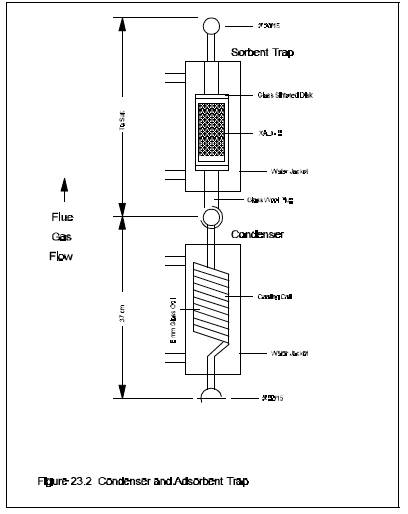
2.1.4 condenser. glass, coil type with compatible fittings. A schematic diagram is shown in Figure 23-2.
2.1.5 Water Bath. Thermostatically controlled to maintain the gas temperature exiting the condenser at 20C (68F).
2.1.6 Adsorbent Module. glass container to hold the solid adsorbent. A schematic diagram is shown in Figure 23-2. Other physical configurations of the resin trap/condenser assembly are acceptable. The connecting fittings shall form leak-free, vacuum tight seals. No sealant greases shall be used in the sampling train. A coarse glass frit is included to retain the adsorbent.
2.2 Sample Recovery.
2.2.1 Fitting Caps. Ground glass, Teflon tape, or aluminum foil (Section 2.2.6) to cap off the sample exposed sections of the train and sorbent module.
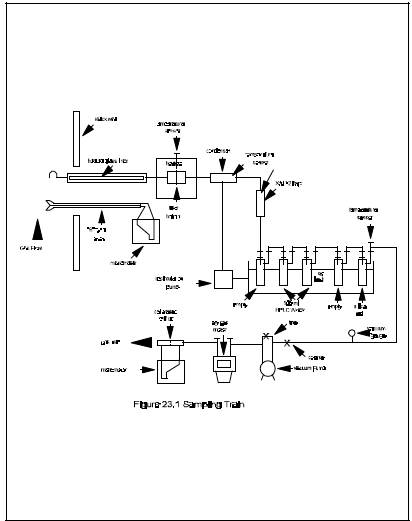
Figure 23.2. condenser and Absorbent Trap
2.2.2 Wash Bottles. Teflon, 500-mL.
2.2.3 Probe Liner, Probe probe nozzle, and Filter Holder Brushes. Inert bristle brushes with pre-cleaned stainless steel or Teflon handles. The Probe brush shall have extensions of stainless steel or Teflon, at least as long as the Probe. The brushes shall be properly sized and shaped to brush out the probe nozzle, Probe liner, and transfer line, if used.
2.2.4 Filter Storage Container. Sealed filter holder, wide-mouth amber glass jar with Teflon-lined cap, glass Petri dish.
2.2.5 Balance. Triple beam.
2.2.6 Aluminum Foil. Heavy duty, hexane-rinsed.
2.2.7 Metal Storage Container. Air tight container to store silica gel.
2.2.8 Graduated Cylinder. glass, 250-mL with 2-mL graduations.
2.2.9 glass Sample Storage Containers. Amber glass bottles for sample glassware washes, 500- or 1000-mL, with leak free Teflon-lined caps.
2.3 Analysis.
2.3.1 Sample Containers. 125- and 250-mL flint glass bottles with Teflon-lined caps.
2.3.2 Test Tubes. glass.
2.3.3 Soxhlet Extraction Apparatus. Capable of holding 43 x 123 mm extraction thimbles.
2.3.4 Extraction Thimble. glass, pre-cleaned cellulosic, or glass fiber.
2.3.5 Pasteur Pipettes. For preparing liquid chromatographic columns.
2.3.6 Reacti-vials. Amber glass, 2-mL, silanized prior to use.
2.3.7 Rotary Evaporator. Buchi/Brinkman RF-121 or equivalent.
2.3.8 Nitrogen Evaporative Concentrator. N-Evap Analytical Evaporator Model III or equivalent.
2.3.9 Separatory Funnels. glass, 2-liter.
2.3.10 Gas Chromatograph. Consisting of the following components:
2.3.10.1 oven. Capable of maintaining the separation column at the proper operating temperature +10C and performing programmed increases in temperature at rates of at least 40C/min.
2.3.10.2 temperature gauges. To monitor column oven, detector, and exhaust temperatures +1C.
2.3.10.3 flow Systems. Gas metering system to measure sample, fuel, combustion gas, and carrier gas flows.
2.3.10.4 Capillary Columns. A fused silica column, 60 x 0.25 mm inside diameter (ID), coated with DB-5 and a fused silica column, 30 m x 0.25 mm ID coated with DB-225. Other column systems may be substituted provided that the user is able to demonstrate, using calibration and performance checks, that the column system is able to meet the specifications of Section 6.1.2.2.
2.3.11 Mass Spectrometer. Capable of routine operation at a resolution of 1:10000 with a stability of +5 ppm.
2.3.12 Data System. Compatible with the mass spectrometer and capable of monitoring at least five groups of 25 ions.
2.3.13 Analytical Balance. To measure within 0.1 mg.
3. REAGENTS
3.1 Sampling.
3.1.1 Filters.
glass fiber filters, without organic binder, exhibiting at least 99.95 percent efficiency (<0.05 percent penetration) on 0.3-micron dioctyl phthalate smoke particles. The filter efficiency test shall be conducted in accordance with ASTM Standard Method D 2986-71 (Reapproved 1978) (incorporated by reference - see 60.17).
3.1.1.1 Pre-cleaning. All filters shall be cleaned before their initial use. Place a glass extraction thimble and 1 g of silica gel and a plug of glass wool into a Soxhlet apparatus, charge the apparatus with toluene, and reflux for a minimum of 3 hours. Remove the toluene and discard it, but retain the silica gel. Place no more than 50 filters in the thimble onto the silica gel bed and top with the cleaned glass wool. Charge the Soxhlet with toluene and reflux for 16 hours. After extraction, allow the Soxhlet to cool, remove the filters, and dry them under a clean nitrogen (N2) stream. Store the filters in a glass Petri dish sealed with Teflon tape.
3.1.2 Adsorbent Resin.
Amberlite XAD-2 resin. Thoroughly cleaned before initial use.
3.1.2.1 Cleaning. Procedure may be carried out in a giant Soxhlet extractor. An all-glass filter thimble containing an extra-coarse frit is used for extraction of XAD-2. The frit is recessed 10-15 mm above a crenellated ring at the bottom of the thimble to facilitate drainage. The resin must be carefully retained in the extractor cup with a glass wool plug and a stainless steel ring because it floats on methylene chloride. This process involves sequential extraction in the following order.

3.1.2.2 Drying.
3.1.2.2.1 Drying Column. Pyrex pipe, 10.2 cm ID by 0.6 m long, with suitable retainers.
3.1.2.2.2 Procedure. The adsorbent must be dried with clean inert gas. Liquid nitrogen from a standard commercial liquid nitrogen cylinder has proven to be a reliable source for large volumes of gas free from organic contaminants. Connect the liquid nitrogen cylinder to the column by a length of cleaned copper tubing, 0.95 cm ID, coiled to pass through a heat source. A convenient heat source is a water-bath heated from a steam line. The final nitrogen temperature should only be warm to the touch and not over 40C. Continue flowing nitrogen through the adsorbent until all the residual solvent is removed. The flow rate should be sufficient to gently agitate the particles, but not so excessive as to cause the particles to fracture.
3.1.2.3 Quality Control Check. The adsorbent must be checked for residual toluene prior to use.
3.1.2.3.1 Extraction. Weigh a 1.0 g sample of dried resin into a small vial, add 3 mL of toluene, cap the vial, and shake it well.
3.1.2.3.2 Analysis. Inject a 2 • l sample of the extract into a gas chromatograph operated under the following conditions:
Column: 6 ft x 1/8 in stainless steel containing 10 percent OV-101™ on 100/120 Supelcoport.
Carrier Gas: Helium at a rate of 30 mL/min.
Detector: Flame ionization detector operated at a sensitivity of 4 x 10-11 A/mV.
Injection Port temperature: 250C.
Detector temperature: 305C.
oven temperature: 30C for 4 min; programmed to rise at 40C/min until it reaches 250C; return to 30C after 17 minutes.
Compare the results of the analysis to the results from the reference solution. Prepare the reference solution by injecting 2.5 • l of methylene chloride into 100 mL of toluene. This corresponds to 100 • g of methylene chloride per g of adsorbent. The maximum acceptable concentration is 1000 • g/g of adsorbent. If the adsorbent exceeds this level, drying must be continued until the excess methylene chloride is removed.
3.1.2.4 Storage. The adsorbent must be used within 4 weeks of cleaning. After cleaning, the adsorbent may be stored in a wide mouth amber glass container with a Teflon-lined cap or placed in glass adsorbent modules tightly sealed with glass stoppers. If pre-cleaned adsorbent is purchased in sealed containers, it must be used within 4 weeks after the seal is broken.
3.1.3 glass Wool.
Cleaned by sequential immersion in three aliquots of methylene chloride, dried in a 110C oven, and stored in a methylene chloride-washed glass container with a Teflon-lined screw cap.
3.1.4 Water.
Deionized distilled and stored in a methylene chloride-rinsed glass container with a Teflon-lined screw cap.
3.1.5 Silica Gel.
Indicating type, 6 to 16 mesh. If previously used, dry at 175 C (350F) for two hours. New silica gel may be used as received. Alternatively, other types of desiccants (equivalent or better) may be used, subject to the approval of the Administrator.
3.1.6 Chromic Acid Cleaning Solution.
Dissolve 20 g of sodium dichromate in 15 mL of water, and then carefully add 400 mL of concentrated sulfuric acid.
3.2 Sample Recovery.
3.2.1 Acetone. Pesticide quality.
3.2.2 Methylene Chloride. Pesticide quality.
3.2.3 Toluene. Pesticide quality.
3.3 Analysis.
3.3.1 Potassium Hydroxide. ACS grade, 2-percent (weight/volume) in water.
3.3.2 Sodium Sulfate. Granulated, reagent grade. Purify prior to use by rinsing with methylene chloride and oven drying. Store the cleaned material in a glass container with a Teflon-lined screw cap.
3.3.3 Sulfuric Acid. Reagent grade.
3.3.4 Sodium Hydroxide. 1.0 N. Weigh 40 g of sodium hydroxide into a 1-liter volumetric flask. Dilute to 1 liter with water.
3.3.5 Hexane. Pesticide grade.
3.3.6 Methylene Chloride. Pesticide grade.
3.3.7 Benzene. Pesticide grade.
3.3.8 Ethyl Acetate.
3.3.9 Methanol. Pesticide grade.
3.3.10 Toluene. Pesticide grade.
3.3.11 Nonane. Pesticide grade.
3.3.12 Cyclohexane. Pesticide Grade.
3.3.13 Basic Alumina. Activity grade 1, 100-200 mesh. Prior to use, activate the alumina by heating for 16 hours at 130C. Store in a desiccator. Pre-activated alumina may be purchased from a supplier and may be used as received.
3.3.14 Silica Gel. Bio-Sil A, 100-200 mesh. Prior to use, activate the silica gel by heating for at least 30 minutes at 180C. After cooling, rinse the silica gel sequentially with methanol and methylene chloride. Heat the rinsed silica gel at 50C for 10 minutes, then increase the temperature gradually to 180C over 25 minutes and maintain it at this temperature for 90 minutes. Cool at room temperature and store in a glass container with a Teflon-lined screw cap.
3.3.15 Silica Gel Impregnated with Sulfuric Acid. Combine 100 g of silica gel with 44 g of concentrated sulfuric acid in a screw capped glass bottle and agitate thoroughly. Disperse the solids with a stirring rod until a uniform mixture is obtained. Store the mixture in a glass container with a Teflon-lined screw cap.
3.3.16 Silica Gel Impregnated with Sodium Hydroxide. Combine 39 g of 1 N sodium hydroxide with 100 g of silica gel in a screw capped glass bottle and agitate thoroughly. Disperse solids with a stirring rod until a uniform mixture is obtained. Store the mixture in glass container with a Teflon-lined screw cap.
3.3.17 Carbon/Celite. Combine 10.7 g of AX-21 carbon with 124 g of Celite 545 in a 250- mL glass bottle with a Teflon-lined screw cap. Agitate the mixture thoroughly until a uniform mixture is obtained. Store in the glass container.
3.3.18 Nitrogen. Ultra high purity.
3.3.19 Hydrogen. Ultra high purity.
3.3.20 Internal Standard Solution. Prepare a stock standard solution containing the isotopic ally labeled PCDD's and PCDF's at the concentrations shown in Table 1 under the heading "Internal Standards" in 10 mL of nonane.
3.3.21 Surrogate Standard Solution. Prepare a stock standard solution containing the isotopic ally labeled PCDD's and PCDF's at the concentrations shown in Table 1 under the heading "Surrogate Standards" in 10 mL of nonane.
3.3.22 Recovery Standard Solution. Prepare a stock standard solution containing the isotopic ally labeled PCDD's and PCDF's at the concentrations shown in Table 1 under the heading "Recovery Standards" in 10 mL of nonane.
4. PROCEDURE
4.1 Sampling.
The complexity of this method is such that, in order to obtain reliable results, testers and analysts should be trained and experienced with the procedures.
4.1.1 Pretest Preparation.
4.1.1.1 Cleaning glassware. All glass components of the train upstream of and including the adsorbent module, shall be cleaned as described in Section 3A of the "Manual of Analytical Methods for the Analysis of Pesticides in Human and Environmental Samples." Special care shall be devoted to the removal of residual silicone grease sealants on ground glass connections of used glassware. Any residue shall be removed by soaking the glassware for several hours in a chromic acid cleaning solution prior to cleaning as described above.
4.1.1.2 Adsorbent Trap. The traps must be loaded in a clean area to avoid contamination. They may not be loaded in the field. Fill a trap with 20 to 40 g of XAD-2. Follow the XAD-2 with glass wool and tightly cap both ends of the trap. Add 100 • l of the surrogate standard solution (Section 3.3.21) to each trap.
4.1.1.3 Sampling Train. It is suggested that all components be maintained according to the procedure described in APTD-0576.
4.1.1.4 Silica Gel. Weigh several 200 to 300 g portions of silica gel in airtight containers to the nearest 0.5 g. Record the total weight of the silica gel plus container, on each container. As an alternative, the silica gel may be weighed directly in its impinger or sampling holder just prior to sampling.
4.1.1.5 Filter. Check each filter against light for irregularities and flaws or pinhole leaks. Pack the filters flat in a clean glass container.
4.1.2 Preliminary Determinations. Same as Section 4.1.2 of Method 5.
4.1.3 Preparation of Collection Train.
4.1.3.1 During preparation and assembly of the sampling train, keep all train openings where contamination can enter, sealed until sampling is about to begin.
4.1.3.2 Place approximately 100 mL of water in the second and third impingers, leave the first and fourth impingers empty, and transfer approximately 200 to 300 g of pre-weighed silica gel from its container to the fifth impinger.
4.1.3.3 Place the silica gel container in a clean place for later use in the sample recovery. Alternatively, the weight of the silica gel plus the fifth impinger may be determined to the nearest 0.5 g and recorded.
4.1.3.4 Assemble the sampling train as shown in Figure 23-1.
4.1.3.5 Turn on the adsorbent module and condenser coil recirculating pump and begin monitoring the adsorbent module gas entry temperature. Ensure proper sorbent gas entry temperature before proceeding and before sampling is initiated. It is extremely important that the XAD-2 adsorbent resin temperature never exceed 50C because thermal decomposition will occur. During testing, the XAD-2 temperature must not exceed 20C for efficient capture of the PCDD's and PCDF's.
4.1.4 Leak-Check Procedure. Same as Method 5, Section 4.1.4.
4.1.5 Sampling Train Operation. Same as Method 5, Section 4.1.5.
4.2 Sample Recovery.
Proper cleanup procedure begins as soon as the Probe is removed from the stack at the end of the sampling period. Seal the probe nozzle end of the sampling Probe with Teflon tape or aluminum foil. When the Probe can be safely handled, wipe off all external particulate matter near the tip of the Probe. Remove the Probe from the train and close off both ends with aluminum foil. Seal off the inlet to the train with Teflon tape, a ground glass cap, or aluminum foil. Transfer the Probe and impinger assembly to the cleanup area. This area shall be clean and enclosed so that the chances of losing or contaminating the sample are minimized. Smoking, which could contaminate the sample, shall not be allowed in the cleanup area. Inspect the train prior to and during disassembly and note any abnormal conditions, e.g., broken filters, colored impinger liquid, etc. Treat the samples as follows:
4.2.1 Container No. 1. Either seal the filter holder or carefully remove the filter from the filter holder and place it in its identified container. Do not place the filter in aluminum foil. Use a pair of cleaned tweezers to handle the filter. If it is necessary to fold the filter, do so such that the particulate cake is inside the fold. Carefully transfer to the container any particulate matter and filter fibers which adhere to the filter holder gasket, by using a dry inert bristle brush and a sharp-edged blade. Seal the container.
4.2.2 Adsorbent Module. Remove the module from the train, tightly cap both ends, label it, and store it on ice for transport to the laboratory.
4.2.3 Container No. 2. Quantitatively recover material deposited in the probe nozzle, Probe transfer lines, the front half of the filter holder, and the cyclone, if used, first, by brushing while rinsing three times with acetone and then, by rinsing the Probe three times with methylene chloride. Collect all the rinses in Container No. 2. Rinse the back half of the filter holder three times with acetone. Rinse the connecting line between the filter and the condenser three times with acetone. Soak the connecting line with three separate portions of methylene chloride for 5 minutes each. If using a separate condenser and adsorbent trap, rinse the condenser in the same manner as the connecting line. Collect all the rinses in Container No. 2 and mark the level of the liquid on the container.
4.2.4 Container No. 3. Repeat the methylene chloride-rinsing described in Section 4.2.3 using toluene as the rinse solvent. Collect the rinses in Container No. 3 and mark the level of the liquid on the container.
4.2.5 impinger Water. Measure the liquid in the first four impingers to within 1 mL by using a graduated cylinder or by weighing it to within 0.5 g by using a balance. Record the volume or weight of liquid present. This information is required to calculate the moisture content of the effluent gas. Discard the liquid after measuring and recording the volume or weight.
4.2.7 Silica Gel. Note the color of the indicating silica gel to determine if it has been completely spent and make a mention of its condition. Transfer the silica gel from the fifth impinger to its original container and seal.
5. ANALYSIS
All glassware shall be cleaned as described in Section 3A of the "Manual of Analytical Methods for the Analysis of Pesticides in Human and Environmental Samples." All samples must be extracted within 30 days of collection and analyzed within 45 days of extraction.
5.1 Sample Extraction.
5.1.1 Extraction System. Place an extraction thimble (Section 2.3.4), 1 g of silica gel, and a plug of glass wool into the Soxhlet apparatus, charge the apparatus with toluene, and reflux for a minimum of 3 hours. Remove the toluene and discard it, but retain the silica gel. Remove the extraction thimble from the extraction system and place it in a glass beaker to catch the solvent rinses.
5.1.2 Container No. 1 (Filter). Transfer the contents directly to the glass thimble of the extraction system and extract them simultaneously with the XAD-2 resin.
5.1.3 Adsorbent Cartridge. Suspend the adsorbent module directly over the extraction thimble in the beaker (See Section 5.1.1). The glass frit of the module should be in the up position. Using a Teflon squeeze bottle containing toluene, flush the XAD-2 into the thimble onto the bed of cleaned silica gel. Thoroughly rinse the glass module catching the rinsings in the beaker containing the thimble. If the resin is wet, effective extraction can be accomplished by loosely packing the resin in the thimble. Add the XAD-2 glass wool plug to the thimble.
5.1.4 Container No. 2 (Acetone and Methylene Chloride). Concentrate the sample to a volume of about 1-2 mL using the rotary evaporator apparatus at a temperature of less than 37C. Rinse the sample container three times with small portions of methylene chloride and add these to the concentrated solution and concentrate further to near dryness. This residue contains particulate matter removed in the rinse of the sampling train Probe and probe nozzle. Add the concentrate to the filter and the AD-2 resin in the Soxhlet apparatus described in Section 5.1.1.
5.1.5 Extraction. Add100 • l of the internal standard solution (Section 3.3.20) to the extraction thimble containing the contents of the adsorbent cartridge, the contents of Container No. 1, and the concentrate from Section 5.1.4. Cover the contents of the extraction thimble with the cleaned glass wool plug to prevent the XAD-2 resin from floating into the solvent reservoir of the extractor. Place the thimble in the extractor, and add the toluene contained in the beaker to the solvent reservoir. Pour additional toluene to fill the reservoir approximately 2/3 full. Add Teflon boiling chips and assemble the apparatus. Adjust the heat source to cause the extractor to cycle three times per hour. Extract the sample for 16 hours. After extraction, allow the Soxhlet to cool. Transfer the toluene extract and three 10-mL rinses to the rotary evaporator. Concentrate the extract to approximately 10 mL. At this point the analyst may choose to split the sample in half. If so, split the sample, store one half for future use, and analyze the other half according to the procedures in Sections 5.2 and 5.3. In either case, use a nitrogen evaporative concentrator to reduce the volume of the sample being analyzed to near dryness. Dissolve the residue in 5 mL of hexane.
5.1.6 Container No. 3 (Toluene Rinse). Add 100 • l of the internal standard solution (Section 3.3.20) to the contents of the container. Concentrate the sample to a volume of about 1-5 mL using the rotary evaporator apparatus at a temperature of less than 37C. Rinse the sample container three times with small portions of toluene and add these to the concentrated solution and concentrate further to near dryness. Analyze the extract separately according to the procedures in Sections 5.2 and 5.3, but concentrate the solution in a rotary evaporator apparatus rather than a nitrogen evaporative concentrator.
5.2 Sample Cleanup and Fractionation.
5.2.1 Silica Gel Column. Pack one end of a glass column, 20 mm x 230 mm, with glass wool. Add in sequence, 1 g silica gel, 2 g of sodium hydroxide impregnated silica gel, 1 g silica gel, 4 g of acid-modified silica gel, and 1 g of silica gel. Wash the column with 30 mL of hexane and discard. Add the sample extract, dissolved in 5 mL of hexane to the column with two additional 5-mL rinses. Elute the column with an additional 90 mL of hexane and retain the entire eluate. Concentrate this solution to a volume of about 1 mL using the nitrogen evaporative concentrator (Section 2.3.8).
5.2.2 Basic Alumina Column. Shorten a 25-mL disposable Pasteur pipette to about 16 mL. Pack the lower section with glass wool and 12 g of basic alumina. Transfer the concentrated extract from the silica gel column to the top of the basic alumina column and elute the column sequentially with 120 mL of 0.5 percent methylene chloride in hexane followed by 120 mL of 35 percent methylene chloride in hexane. Discard the first 120 mL of eluate. Collect the second 120 mL of eluate and concentrate it to about 0.5 mL using the nitrogen evaporative concentrator.
5.2.3 AX-21 Carbon/Celite 545 Column. Remove the bottom 0.5 in. from the tip of a 9-mL disposable Pasteur pipette. Insert a glass fiber filter disk or glass wool plug in the top of the pipette 2.5 cm from the constriction. Add sufficient carbon/Celite™ mixture to form a 2 cm column (the 0.6 mL mark column. Top with a glass wool plug. In some cases AX-21 carbon fines may wash through the glass wool plug and enter the sample. This may be prevented by adding a celite plug to the exit end of the column. Rinse the column in sequence with 2 mL of 50 percent benzene in ethyl acetate, 1 mL of a 50 percent methylene chloride in cyclohexane mixture, and 2 mL of hexane. Discard these rinses. Transfer, the concentrate in 1 mL hexane from the basic alumina column to the carbon/celite along with 1 ml of hexane rinse. Elute the column sequentially with 2 mL of 50 percent methylene chloride in hexane and 2 mL of 50 percent benzene in ethyl acetate and discard the eluates. Invert the column and elute in the reverse direction with 13 mL of toluene. Collect this eluate. Concentrate the eluate in a rotary evaporator at 50C to about 1 mL. Transfer the concentrate to a Reacti-vial using a toluene rinse and concentrate to a volume of 200 l using a stream of N2. Store extracts at room temperature, shielded from light, until the analysis is performed.
5.3 Analysis.
Analyze the sample with a gas chromatograph coupled to a mass spectrometer (GC/MS) using the instrumental parameters in Sections 5.3.1 and 5.3.2. Immediately prior to analysis, add a 20 • l aliquot of the recovery standard solution from Table 1 to each sample. A 2 • l aliquot of the extract is injected into the GC. Sample extracts are first analyzed using the DB-5 capillary column to determine the concentration of each isomer of PCDD's and PCDF's (tetra-through octa-). If tetrachlorinated dibenzofurans are detected in this analysis, then analyze another aliquot of the sample in a separate run, using the DB-225 column to measure the 2,3,7,8 tetra-chloro dibenzofuran isomer. Other column systems may be used, provided that the user is able to demonstrate using calibration and performance checks that the column system is able to meet the specifications of Section 6.1.2.2.
5.3.1 Gas Chromatograph Operating Conditions.
5.3.1.1 Injector. Configured for capillary column, splitless, 250 C.
5.3.1.2 Carrier Gas. Helium, 1-2 ml/min.
5.3.1.3 oven. Initially at 150 C. Raise by at least 40 C/min to 190 C and then by C/min up to 300 C.
5.3.2 High Resolution Mass Spectrometer.
5.3.2.1 Resolution. 10,000 m/e.
5.3.2.2 Ionization Mode. Electron impact.
5.3.2.3 Source temperature 250C.
5.3.2.4 Monitoring Mode. Selected ion monitoring. A list of the various ions to be monitored is presented in Table 3.
5.3.2.5 Identification Criteria. The following identification criteria shall be used for the characterization of polychlorinated dibenzodioxins and dibenzofurans.
1. The integrated ion-abundance ratio (M/M+2 or M+2/M+4) shall be within 15 percent of the theoretical value. The acceptable ion-abundance ratio ranges (+15%) for the identification of chlorine-containing compounds are given in Table 4.
2. The retention time for the analytes must be within 3 seconds of the corresponding 13C-labeled internal standard or surrogate standard.
3. The monitored ions, shown in Table 3 for a given analyte, shall reach their maximum within 2 seconds of each other.
4. The identification of specific isomers that do not have corresponding 13C-labeled standards is done by comparison of the relative retention time (RRT) of the analyte to the nearest internal standard retention time with reference (i.e., within 0.005 RRT units) to the comparable RRT's found in the continuing calibration.
5. The signal to noise ratio for all monitored ions must be greater than 2.5.
6. The confirmation of 2, 3, 7, 8-TCDF shall satisfy all of the above identification criteria.
7. For the identification of PCDF’s, no signal may be found in the corresponding PCDPE channels.
5.3.2.6 Quantification. The peak areas for the two ions monitored for each analyte are summed to yield the total response for each analyte. Each internal standard is used to quantify the indigenous PCDD's or PCDF's in its homologous series. For example, the 13C12-2,3,7,8-tetra chlorinated dibenzodioxin is used to calculate the concentrations of all other tetra chlorinated isomers. Recoveries of the tetra- and penta- internal standards are calculated using the 13C12-1,2,3,4-TCDD. Recoveries of the hexa- through octa- internal standards are calculated using 13C12-1,2,3,7,8,9-HxCDD. Recoveries of the surrogate standards are calculated using the corresponding homolog from the internal standard.
6. calibration
Same as Method 5 with the following additions.
6.1 GC/MS System.
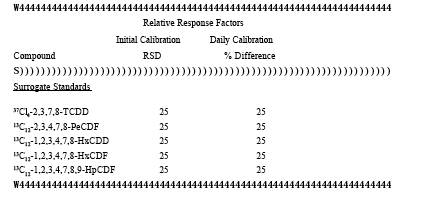
6.1.1 Initial calibration. Calibrate the GC/MS system using the set of five standards shown in Table 2. The relative standard deviation for the mean response factor from each of the unlabeled analytes (Table 2) and of the internal and surrogate standards shall be less than or equal to the values in Table 5. The signal to noise ratio for the GC signal present in every selected ion current profile shall be greater than or equal to 2.5. The ion abundance ratios shall be within the control limits in Table 4.
6.1.2 Daily Performance Check.
6.1.2.1 calibration Check. Inject one • l of solution Number 3 from Table 2. Calculate the relative response factor (RRF) for each compound and compare each RRF to the corresponding mean RRF obtained during the initial calibration. The analyzer performance is acceptable if the measured RRF's for the labeled and unlabeled compounds for the daily run are within the limits of the mean values shown in Table 5. In addition, the ion-abundance ratios shall be within the allowable control limits shown in Table 4.
6.1.2.2 Column Separation Check. Inject a solution of a mixture of PCDD's and PCDF's that documents resolution between 2,3,7,8-TCDD and other TCDD isomers. Resolution is defined as a valley between peaks that is less than 25 percent of the lower of the two peaks. Identify and record the retention time windows for each homologous series. Perform a similar resolution check on the confirmation column to document the resolution between 2,3,7,8 TCDF and other TCDF isomers.
6.2 Lock Channels.
Set mass spectrometer lock channels as specified in Table 3. Monitor the quality control check channels specified in Table 3 to verify instrument stability during the analysis.
7. QUALITY CONTROL
7.1 Sampling Train Collection Efficiency Check.
Add 100 • l of the surrogate standards in Table 1 to the adsorbent cartridge of each train before collecting the field samples.
7.2 Internal Standard Percent Recoveries.
A group of nine carbon-labeled PCDDs and PCDFs representing the tetra- through octachlorinated homologues, is added to every sample prior to extraction. The role of the internal standards is to quantify the native PCDD's and PCDF's present in the sample as well as to determine the overall method efficiency. Recoveries of the internal standards must be between 40 to 130 percent for the tetra- through hexachlorinated compounds while the range is 25 to 130 percent for the hepta- and octachlorinated homologues.
7.3 Surrogate Standard Recoveries.
The five surrogate compounds in Table 2 are added to the resin in the adsorbent sampling cartridge before the sample is collected. The surrogate recoveries are measured relative to the internal standards and are a measure of the collection efficiency. They are not used to measure the native PCDD's and PCDF's. All recoveries shall be between 70 and 130 percent. Poor recoveries for all the surrogates may be an indication of breakthrough in the sampling train. If the recovery of all standards is below 70 percent, the sampling runs must be repeated. As an alternative, the sampling runs do not have to be repeated if the final results are divided by the fraction of surrogate recovery. Poor recoveries of isolated surrogate compounds should not be grounds for rejecting an entire set of samples.
7.4 Toluene QA Rinse.
Report the results of the toluene QA rinse separately from the total sample catch. Do not add it to the total sample.
8. QUALITY ASSURANCE
8.1 Applicability.
When the method is used to analyze samples to demonstrate compliance with a source emission regulation, an audit sample must be analyzed, subject to availability.
8.2 Audit Procedure.
Analyze an audit sample with each set of compliance samples. The audit sample contains tetra through octa isomers of PCDD and PCDF. Concurrently analyze the audit sample and a set of compliance samples in the same manner to evaluate the technique of the analyst and the standards preparation. The same analyst, analytical reagents, and analytical system shall be used both for the compliance samples and the EPA audit sample.
8.3 Audit Sample Availability.
Audit samples will be supplied only to enforcement agencies for compliance tests. Audit samples may be obtained by writing:
Source Test Audit Coordinator (MD-77B)
Quality Assurance Division
Atmospheric Research and Exposure Assessment Laboratory
U.S. Environmental Protection Agency
Research Triangle Park, NC 27711
or by calling the Source Test Audit Coordinator (STAC) at (919) 541-7834. The audit sample request must be made at least 30 days prior to the scheduled compliance sample analysis.
8.4 Audit Results.
Calculate the audit sample concentration according to the calculation procedure provided in the audit instructions included with the audit sample. Fill in the audit sample concentration and the analyst's name on the audit response form included with the audit instructions. Send one copy to the EPA Regional Office or the appropriate enforcement agency and a second copy to the STAC. The EPA Regional office or the appropriate enforcement agency will report the results of the audit to the laboratory being audited. Include this response with the results of the compliance samples in relevant reports to the EPA Regional Office or the appropriate enforcement agency.
9. CALCULATIONS
Same as Method 5, Section 6 with the following additions.
9.1 Nomenclature.

9.2 Average Relative Response Factor.

9.3 Concentration of the PCDD's and PCDF's.

9.4 Recovery Standard Response Factor.

9.5 Recovery of Internal Standards (R*).

9.6 Surrogate Compound Response Factor.

9.7 Recovery of Surrogate Compounds (Rs).

9.8 Minimum Detectable Limit (DL).

9.9 Total Concentration of PCDD's and PCDF's in the Sample.

Any PCDDs or PCDFs that are reported as nondetected (below the DL) shall be counted as zero for the purpose of calculating the total concentration of PCDDs and PCDFs in the sample.
10. BIBLIOGRAPHY
1. American Society of Mechanical Engineers. Sampling for the Determination of Chlorinated Organic Compounds in Stack Emissions. Prepared for U.S. Department of Energy and U.S. Environmental Protection Agency. Washington DC. December 1984. 25 p.
2. American Society of Mechanical Engineers. Analytical Procedures to Assay Stack Effluent Samples and Residual Combustion Products for Polychlorinated Dibenzo-p-Dioxins (PCDD) and Polychlorinated Dibenzofurans (PCDF). Prepared for the U.S. Department of Energy and U.S. Environmental Protection Agency. Washington, DC. December 1984. 23 p.
3. Thompson, J. R. (ed.). Analysis of Pesticide Residues in Human and Environmental Samples. U.S. Environmental Protection Agency. Research Triangle Park, NC. 1974.
4. Triangle Laboratories. Case Study: Analysis of Samples for the Presence of Tetra Through Octachloro-p-Dibenzodioxins and Dibenzofurans. Research Triangle Park, NC. 1988. 26 p.
5. U.S. Environmental Protection Agency. Method 8290 - The Analysis of Polychlorinated Dibenzo-p-dioxin and Polychlorinated Dibenzofurans by High-Resolution Gas Chromatography/High-Resolution Mass Spectrometry. In: Test Methods for Evaluating Solid Waste. Washington, DC. SW-846.
TABLE 23-1. COMPOSITION OF THE SAMPLE FORTIFICATION AND RECOVERY STANDARDS SOLUTIONS

TABLE 23-2. COMPOSITION OF THE INITIAL calibration SOLUTIONS
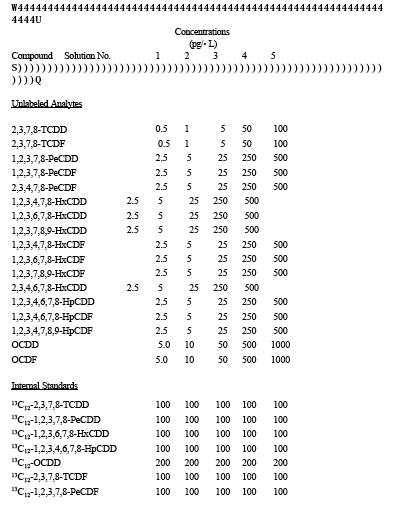

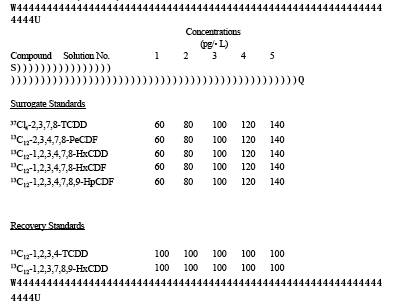
TABLE 23-3. ELEMENTAL COMPOSITIONS AND EXACT MASSES OF THE IONS MONITORED BY HIGH RESOLUTION MASS SPECTROMETRY FOR PCDD's AND PCDF's
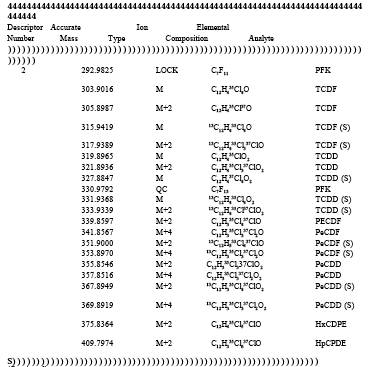

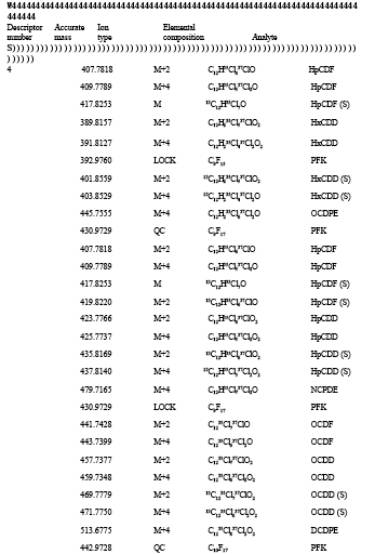
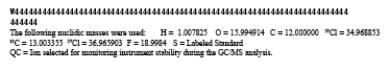
TABLE 23-4. ACCEPTABLE RANGES FOR ION-ABUNDANCE RATIOS OF PCDD's AND PCDF's
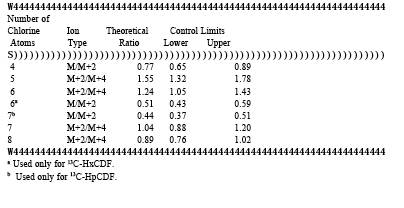
TABLE 23-5. MINIMUM REQUIREMENTS FOR INITIAL AND DAILY calibration RESPONSE FACTORS

- Analytical
- Ion Chromatography
- Gas Chromatography
- Gravimetrics
- Ash Resistivity
- Inks/Coatings
- Scrubber Stoichiometry
- Titrations
- Mercury Sorbent Trap
- Engineering
- Express Products
- Rental Instruments
- MET80 Mercury Monitor
- Continuous Emission Monitors
- Gas Sampling Equipment
- Mobile Power Supply
Our Resources
- Technical Resources

 Express
Express FTIR
FTIR Mercury
Mercury Emission Sampling Equipment
Emission Sampling Equipment Instrument Rental
Instrument Rental