EPA Methods List with Links
US EPA Method 105 - Determination Of Mercury In Wastewater Treatment Plant Sewage Sludges
NOTE: This method does not include all of the specifications (e.g., equipment and supplies) and procedures (e.g., sampling and analytical) essential to its performance. Some material is incorporated by reference from other methods in this part. Therefore, to obtain reliable results, persons using this method should also have a thorough knowledge of at least the following additional test methods: Method 101 and Method 101A.
1.0 Scope and Application.
1.1 Analytes.
| Analyte | CAS No. | Sensitivity |
| Mercury (Hg) | 7439-97-6 | Dependent upon spectrophotometer and recorder |
1.2 Applicability.
This method is applicable for the determination of total organic and inorganic Hg content in sewage sludges.
1.3 Data Quality Objectives.
Adherence to the requirements of this method will enhance the quality of the data obtained from air pollutant sampling methods.
2.0 Summary of Method.
2.1 Time-composite sludge samples are withdrawn from the conveyor belt subsequent to dewatering and before incineration or drying. A weighed portion of the sludge isdigested in aqua regia and is oxidized by potassium permanganate (KMnO4). Mercury in the digested sample is then measured by the conventional spectrophotometric cold- vapor technique.
3.0 Definitions. [Reserved]
4.0 Interferences. [Reserved]
5.0 Safety.
5.1 Disclaimer.
This method may involve hazardous materials, operations, and equipment. This test method may not address all of the safety problems associated with its use. It is the responsibility of the user of this test method to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to performing this test method.
5.2 Corrosive Reagents.
The following reagents are hazardous. Personal protective equipment and safe procedures are useful in preventing chemical splashes. If contact occurs, immediately flush with copious amounts of water at least 15 minutes. Remove clothing under shower and decontaminate. Treat residual chemical burn as thermal burn.
5.2.1 Hydrochloric Acid (HCl).
Highly toxic. Vapors are highly irritating to eyes, skin, nose, and lungs, causing severe damage. May cause bronchitis, pneumonia, or edema of lungs. Exposure to concentrations of 0.13 to 0.2 percent can be lethal to humans in a few minutes. Provide ventilation to limit exposure. Reacts with metals, producing hydrogen gas.
5.2.2 Nitric Acid (HNO3).
Highly corrosive to eyes, skin, nose, and lungs. Vapors cause bronchitis, pneumonia, or edema of lungs. Reaction to inhalation may be delayed as long as 30 hours and still be fatal. Provide ventilation to limit exposure. Strong oxidizer. Hazardous reaction may occur with organic materials such as solvents.
6.0 Equipment and Supplies.
6.1 Sample Collection and Mixing.
The following items are required for collection and mixing of the sludge samples:
6.1.1 Container. Plastic, 50-liter.
6.1.2 Scoop. To remove 950-ml (1 quart.) sludge sample.
6.1.3 Mixer. Mortar mixer, wheelbarrow-type, 57 liter (or equivalent) with electricity-driven motor.
6.1.4 Blender. Waring-type, 2-liter.
6.1.5 Scoop. To remove 100-ml and 20-ml samples of blended sludge.
6.1.6 Erlenmeyer Flasks. Four, 125-ml.
6.1.7 Beakers. glass beakers in the following sizes: 50 ml (1), 200 ml (1), 400 ml (2).
6.2 Sample Preparation and Analysis.
Same as Method 101, Section 6.3, with the addition of the following:
6.2.1 Hot Plate.
6.2.2 Desiccator.
6.2.3 filter Paper. S and S No. 588 (or equivalent).
6.2.4 Beakers. glass beakers, 200 ml and 400 ml (2 each).
7.0 Reagents and Standards.
NOTE: Unless otherwise indicated, it is intended that all reagents conform to the specifications established by the Committee on Analytical Reagents of the American Chemical Society, where such specifications are available; otherwise, use the best available grade.
7.1 Sample Analysis.
Same as Method 101A, Section 7.2, with the following additions and exceptions:
7.1.1 Hydrochloric Acid.
The concentrated HCl specified in Method 101A, Section 7.2.4, is not required.
7.1.2 Aqua Regia.
Prepare immediately before use. Carefully add one volume of concentrated HNO3 to three volumes of concentrated HCl.
8.0 Sample Collection, Preservation, Storage, and Transport.
8.1 Sludge Sampling.
Withdraw equal volume increments of sludge [for a total of at least 15 liters (16 quarts)] at intervals of 30 min over an 8-hr period, and combine in a rigid plastic container.
8.2 Sludge Mixing.
Transfer the entire 15-liter sample to a mortar mixer. Mix the sample for a minimum of 30 min at 30 rpm. Take six 100-ml portions of sludge, and combine in a 2-liter blender. Blend sludge for 5 min; add water as necessary to give a fluid consistency. Immediately after stopping the blender, withdraw four 20-ml portions of blended sludge, and place them in separate, tared 125-ml Erlenmeyer flasks. Reweigh each flask to determine the exact amount of sludge added.
8.3 Sample Holding Time.
Samples shall be analyzed within the time specified in the applicable subpart of the regulations.
9.0 Quality Control.
| Section | Quality Control Measure | Effect |
| 10.0 | Spectrophotometer calibration | Ensure linearity of spectrophotometer response to standards |
| 11.0 | Check for matrix effects | Eliminate matrix effects |
10.0 Calibration and Standardization.
Same as Method 101A, Section 10.2.
11.0 Analytical Procedures.
11.1 Solids Content of Blended Sludge.
Dry one of the 20-ml blended samples from Section 8.2 in an oven at 105 °C (221 °F) to constant weight. Cool in a desiccator, weigh and record the dry weight of the sample.
11.2 Aqua Regia Digestion of Blended Samples.
11.2.1 To each of the three remaining 20-ml samples from Section 8.2 add 25 ml of aqua regia, and digest the on a hot plate at low heat (do not boil) for 30 min, or until samples are a pale yellow-brown color and are void of the dark brown color characteristic of organic matter. Remove from hotplate and allow to cool.
11.2.2 filter each digested sample separately through an S and S No. 588 filter or equivalent, and rinse the filter contents with 50 ml of water. Transfer the filtrate and filter washing to a 100-ml volumetric flask, and carefully dilute to volume with water.
11.3 Solids Content of the Sludge Before Blending.
Remove two 100-ml portions of mixed sludge from the mortar mixer and place in separate, tared 400-ml beakers. Reweigh each beaker to determine the exact amount of sludge added. Dry in oven at 105 °C (221 °F) and cool in a desiccator to constant weight.
11.4 Analysis for Mercury.
Analyze the three aqua regia-digested samples using the procedures outlined in Method 101A, Section 11.0.
12.0 Data Analysis and Calculations.
12.1 Nomenclature.
| Cm | = | Concentration of Hg in the digested sample, μg/g. |
| Fsb | = | Weight fraction of solids in the blended sludge. |
| Fsm | = | Weight fraction of solids in the collected sludge after mixing. |
| M | = | Hg content of the sewage sludge (on a dry basis), μg/g. |
| m | = | Mass of Hg in the aliquot of digested sample analyzed, μg. |
| n | = | number of digested samples (specified in Section 11.2 as three). |
| Va | = | Volume of digested sample analyzed, ml. |
| Vs | = | Volume of digested sample, ml. |
| Wb | = | Weight of empty sample beaker, g. |
| Wbs | = | Weight of sample beaker and sample, g. |
| Wbd | = | Weight of sample beaker and sample after drying, g. |
| Wf | = | Weight of empty sample flask, g. |
| Wfd | = | Weight of sample flask and sample after drying, g. |
| Wfs | = | Weight of sample flask and sample, g. |
12.2 Mercury Content of Digested Sample (Wet Basis).
12.2.1 For each sample analyzed for Hg content, calculate the arithmetic mean maximum absorbance of the two consecutive samples whose peak heights agree ±3 percent of their average. Correct this average value for the contribution of the blank. Use the calibration curve and these corrected averages to determine the final Hg concentration in the solution cell for each sludge sample.
12.2.2 Calculate the average Hg concentration of the digested samples by correcting for any dilutions made to bring the sample into the working range of the spectrophotometer and for the weight of the sludge portion digested, using Equation 105-1.
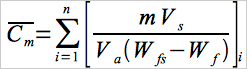 Eq. 105-1
Eq. 105-1
12.3 Solids Content of Blended Sludge.
Determine the solids content of the blended sludge using Equation 105-2.
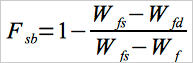 Eq. 105-2
Eq. 105-2
12.4 Solids Content of Bulk Sample (before blending but, after mixing in mortar mixer).
Determine the solids content of each 100 ml aliquot (Section 11.3), and average the results.
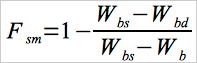 Eq. 105-3
Eq. 105-3
12.5 Mercury Content of Bulk Sample (Dry Basis).
Average the results from the three samples from each 8-hr composite sample, and calculate the Hg concentration of the composite sample on a dry basis.
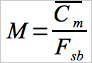 Eq. 105-4
Eq. 105-4
13.0 Method Performance.
13.1 Range.
The range of this method is 0.2 to 5 micrograms per gram; it may be extended by increasing or decreasing sample size.
14.0 Pollution Prevention. [Reserved]
15.0 Waste Management. [Reserved]
16.0 References.
1. Bishop, J.N. Mercury in Sediments. Ontario Water Resources Commission. Toronto, Ontario, Canada. 1971.
2. Salma, M. Private Communication. EPA California/Nevada Basin Office. Alameda, California. 3. Hatch, W.R. and W.L. Ott. Determination of Sub- Microgram Quantities of Mercury by Atomic Absorption Spectrophotometry. Analytical Chemistry. 40:2085. 1968.
4. Bradenberger, H., and H. Bader. The Determination of Nanogram Levels of Mercury in Solution by a Flameless Atomic Absorption Technique. Atomic Absorption Newsletter. 6:101. 1967.
5. Analytical Quality Control Laboratory (AQCL). Mercury in Sediment (Cold Vapor Technique) (Provisional Method). U.S. Environmental Protection Agency. Cincinnati, Ohio. April 1972.
6. Kopp, J.F., M.C. Longbottom, and L.B. Lobring. "Cold Vapor" Method for Determining Mercury. Journal AWWA. 64(1):20-25. 1972.
7. Manual of Methods for Chemical Analysis of Water and Wastes. U.S. Environmental Protection Agency. Cincinnati, Ohio. Publication No. EPA-624/2-74-003. December 1974. pp. 118-138.
8. Mitchell, W.J., M.R. Midgett, J. Suggs, R.J. Velton, and D. Albrink. Sampling and Homogenizing Sewage for Analysis. Environmental Monitoring and Support Laboratory, Office of Research and Development, U.S. Environmental Protection Agency. Research Triangle Park, N.C. March 1979. p. 7.
17.0 Tables, Diagrams, flowcharts, and Validation Data. [Reserved]
- Analytical
- Ion Chromatography
- Gas Chromatography
- Gravimetrics
- Ash Resistivity
- Inks/Coatings
- Scrubber Stoichiometry
- Titrations
- Mercury Sorbent Trap
- Engineering
- Express Products
- Rental Instruments
- MET80 Mercury Monitor
- Continuous Emission Monitors
- Gas Sampling Equipment
- Mobile Power Supply
Our Resources
- Technical Resources

 Express
Express FTIR
FTIR Mercury
Mercury Emission Sampling Equipment
Emission Sampling Equipment Instrument Rental
Instrument Rental