EPA Methods List with Links
US EPA Method 16A - Determination Of Total Reduced Sulfur Emissions From Stationary Sources (Impinger Technique)
NOTE: This method does not include all of the specifications (e.g., equipment and supplies) and procedures (e.g., sampling and analytical) essential to its performance. Some material is incorporated by reference from other methods in this part. Therefore, to obtain reliable results, persons using this method should have a thorough knowledge of at least the following additional test methods: Method 1, Method 6, and Method 16.
Content [ show/hide ].1.0 Scope and Application.
1.1 Analytes.
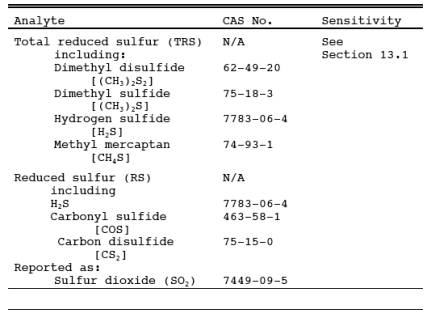
1.2 Applicability.
This method is applicable for the determination of TRS emissions from recovery boilers, lime kilns, and smelt dissolving tanks at kraft pulp mills, reduced sulfur compounds (H2S, carbonyl sulfide, and carbon disulfide from sulfur recovery units at onshore natural gas processing facilities, and from other sources when specified in an applicable subpart of the regulations. The flue gas must contain at least 1 percent oxygen for complete oxidation of all TRS to SO2.
1.3 Data Quality Objectives.
Adherence to the requirements of this method will enhance the quality of the data obtained from air pollutant sampling methods.
2.0 Summary of Method.
2.1 An integrated gas sample is extracted from the stack.
SO2 is removed selectively from the sample using a citrate buffer solution. TRS compounds are then thermally oxidized to SO2, collected in hydrogen peroxide as sulfate, and analyzed by the Method 6 barium-thorin titration procedure.
3.0 Definitions. [Reserved]
4.0 Interferences.
4.1 Reduced sulfur compounds other than those regulated by the emission standards, if present, may be measured by this method.
Therefore, carbonyl sulfide, which is partially oxidized to SO2 and may be present in a lime kiln exit stack, would be a positive interferent.
4.2 Particulate matter from the lime kiln stack gas (primarily calcium carbonate) can cause a negative bias if it is allowed to enter the citrate scrubber; the particulate matter will cause the pH to rise and H2S to be absorbed prior to oxidation. Furthermore, if the calcium carbonate enters the hydrogen peroxide impingers, the calcium will precipitate sulfate ion. Proper use of the particulate filter described in Section 6.1.3 will eliminate this interference.
5.0 Safety.
5.1 Disclaimer.
This method may involve hazardous materials, operations, and equipment. This test method may not address all of the safety problems associated with its use. It is the responsibility of the user of this test method to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to performing this test method.
5.2 Corrosive reagents.
The following reagents are hazardous. Personal protective equipment and safe procedures are useful in preventing chemical splashes. If contact occurs, immediately flush with copious amounts of water for at least 15 minutes. Remove clothing under shower and decontaminate. Treat residual chemical burns as thermal burns.
5.2.1 Hydrogen Peroxide (H2O2). Irritating to eyes, skin, nose, and lungs.
5.2.2 Sodium Hydroxide (NaOH). Causes severe damage to eyes and skin.
Inhalation causes irritation to nose, throat, and lungs. Reacts exothermically with limited amounts of water.
5.2.3 Sulfuric Acid (H2SO4).
Rapidly destructive to body tissue. Will cause third degree burns. Eye damage may result in blindness. Inhalation may be fatal from spasm of the larynx, usually within 30 minutes. May cause lung tissue damage with edema. 3 mg/m3 will cause lung damage in uninitiated. 1 mg/m3 for 8 hours will cause lung damage or, in higher concentrations, death. Provide ventilation to limit inhalation. Reacts violently with metals and organics.
5.3 Hydrogen Sulfide (H2S).
A flammable, poisonous gas with the odor of rotten eggs. H2S is extremely hazardous and can cause collapse, coma, and death within a few seconds of one or two inhalations at sufficient concentrations. Low concentrations irritate the mucous membranes and may cause nausea, dizziness, and headache after exposure.
6.0 Equipment and Supplies.
6.1 Sample Collection.
The sampling train is shown in Figure 16A-1 and component parts are discussed below. Modifications to this sampling train are acceptable provided the system performance check is met (see Section 8.5).
6.1.1 Probe.
Teflon tubing, 6.4-mm (1/4-in.) diameter, sequentially wrapped with heat-resistant fiber strips, a rubberized heat tape (plug at one end), and heat-resistant adhesive tape. A flexible thermocouple or other suitable temperature-measuring device should be placed between the Teflon tubing and the fiber strips so that the temperature can be monitored to prevent softening of the Probe. The Probe should be sheathed in stainless steel to provide in-stack rigidity. A series of bored-out stainless steel fittings placed at the front of the sheath will prevent moisture and particulate from entering between the Probe and sheath. A 6.4-mm (1/4-in.) Teflon elbow (bored out) should be attached to the inlet of the Probe, and a 2.54 cm (1 in.) piece of Teflon tubing should be attached at the open end of the elbow to permit the opening of the Probe to be turned away from the particulate stream; this will reduce the amount of particulate drawn into the sampling train. The Probe is depicted in Figure 16A-2.
6.1.2 Probe Brush.
Nylon bristle brush with handle inserted into a 3.2-mm (c-in.) Teflon tubing. The Teflon tubing should be long enough to pass the brush through the length of the Probe.
6.1.3 Particulate filter.
50-mm Teflon filter holder and a 1- to 2-m porosity, Teflon filter (available through Savillex Corporation, 5325 Highway 101, Minnetonka, Minnesota 55343). The filter holder must be maintained in a hot box at a temperature sufficient to prevent moisture condensation. A temperature of 121 C (250 F) was found to be sufficient when testing a lime kiln under sub-freezing ambient conditions.
6.1.4 SO2 Scrubber.
Three 300-ml Teflon segmented impingers connected in series with flexible, thick-walled, Teflon tubing. (impinger parts and tubing available through Savillex.) The first two impingers contain 100 ml of citrate buffer and the third impinger is initially dry. The tip of the tube inserted into the solution should be constricted to less than 3 mm (c in.) ID and should be immersed to a depth of at least 5 cm (2 in.).
6.1.5 Combustion Tube.
Quartz glass tubing with an expanded combustion chamber 2.54 cm (1 in.) in diameter and at least 30.5 cm (12 in.) long. The tube ends should have an outside diameter of 0.6 cm (1/4 in.) and be at least 15.3 cm (6 in.) long. This length is necessary to maintain the quartz-glass connector near ambient temperature and thereby avoid leaks. Alternatively, the outlet may be constructed with a 90-degree glass elbow and socket that would fit directly onto the inlet of the first peroxide impinger.
6.1.6 Furnace.
A furnace of sufficient size to enclose the combustion chamber of the combustion tube with a temperature regulator capable of maintaining the temperature at 800 ± 100 C (1472 ± 180 F). The furnace operating temperature should be checked with a thermocouple to ensure accuracy.
6.1.7 Peroxide impingers, Stopcock Grease, tenperature sensor, Drying Tube, Valve, pump, and barometer.
Same as Method 6, Sections 6.1.1.2, 6.1.1.4, 6.1.1.5, 6.1.1.6, 6.1.1.7, 6.1.1.8, and 6.1.2, respectively, except that the midget bubbler of Method 6, Section 6.1.1.2 is not required.
6.1.8 Vacuum Gauge.
At least 760 mm Hg (30 in. Hg) gauge.
6.1.9 Rate Meter.
Rotameter, or equivalent, accurate to within 5 percent at the selected flow rate of approximately 2 liters/min (4.2 ft3/hr).
6.1.10 Volume meter.
console meter capable of measuring the sample volume under the sampling conditions of 2 liters/min (4.2 ft3/hr) with an accuracy of 2 percent.
6.2 Sample Recovery.
Polyethylene Bottles, 250-ml (one per sample).
6.3 Sample Preparation and Analysis.
Same as Method 6, Section 6.3, except a 10-ml buret with 0.05-ml graduations is required, and the spectrophotometer is not needed.
7.0 Reagents and Standards.
NOTE: Unless otherwise indicated, all reagents must conform to the specifications established by the Committee on Analytical Reagents of the American Chemical Society. When such specifications are not available, the best available grade must be used.
7.1 Sample Collection.
The following reagents are required for sample analysis:
7.1.1 Water.
Same as in Method 6, Section 7.1.1.
7.1.2 Citrate Buffer.
Dissolve 300 g of potassium citrate (or 284 g of sodium citrate) and 41 g of anhydrous citric acid in 1 liter of water (200 ml is needed per test). Adjust the pH to between 5.4 and 5.6 with potassium citrate or citric acid, as required.
7.1.3 Hydrogen Peroxide, 3 percent.
Same as in Method 6, Section 7.1.3 (40 ml is needed per sample).
7.1.4 Recovery Check Gas.
Hydrogen sulfide (100 ppmv or less) in nitrogen, stored in aluminum cylinders. Verify the concentration by Method 11 or by gas chromatography where the instrument is calibrated with an H2S permeation tube as described below. For Method 11, the relative standard deviation should not exceed 5 percent on at least three 20-minute runs.
NOTE: Alternatively, hydrogen sulfide recovery gas generated from a permeation device gravimetrically calibrated and certified at some convenient operating temperature may be used. The permeation rate of the device must be such that at a dilution gas flow rate of 3 liters/min (6.4 ft3/hr), an H2S concentration in the range of the stack gas or within 20 percent of the standard can be generated.
7.1.5 Combustion Gas.
Gas containing less than 50 ppb reduced sulfur compounds and less than 10 ppmv total hydrocarbons. The gas may be generated from a clean-air system that purifies ambient air and consists of the following components: Diaphragm pump, silica gel drying tube, activated charcoal tube, and flow rate measuring device. flow from a compressed air cylinder is also acceptable.
7.2 Sample Recovery and Analysis.
Same as Method 6, Sections 7.2.1 and 7.3, respectively.
8.0 Sample Collection, Preservation, Storage, and Transport.
8.1 Preparation of Sampling Train.
8.1.1 For the SO2 scrubber, measure 100 ml of citrate buffer into the first and second impingers; leave the third impinger empty.
Immerse the impingers in an ice bath, and locate them as close as possible to the filter heat box. The connecting tubing should be free of loops. Maintain the Probe and filter temperatures sufficiently high to prevent moisture condensation, and monitor with a suitable tenperature sensor.
8.1.2 For the Method 6 part of the train, measure 20 ml of 3 percent hydrogen peroxide into the first and second midget impingers. Leave the third midget impinger empty, and place silica gel in the fourth midget impinger. Alternatively, a silica gel drying tube may be used in place of the fourth impinger. Maintain the oxidation furnace at 800 ± 100 C (1472 ± 180 F). Place crushed ice and water around all impingers.
8.2 Citrate Scrubber Conditioning Procedure.
Condition the citrate buffer scrubbing solution by pulling stack gas through the Teflon impingers and bypassing all other sampling train components. A purge rate of 2 liters/min for 10 minutes has been found to be sufficient to obtain equilibrium. After the citrate scrubber has been conditioned, assemble the sampling train, and conduct (optional) a leak-check as described in Method 6, Section 8.2.
8.3 Sample Collection.
Same as in Method 6, Section 8.3, except the sampling rate is 2 liters/min (± 10 percent) for 1 or 3 hours. After the sample is collected, remove the Probe from the stack, and conduct (mandatory) a post-test leak-check as described in Method 6, Section 8.2. The 15-minute purge of the train following collection should not be performed. After each 3-hour test run (or after three 1-hour samples), conduct one system performance check (see Section 8.5) to determine the reduced sulfur recovery efficiency through the sampling train. After this system performance check and before the next test run, rinse and brush the Probe with water, replace the filter, and change the citrate scrubber (optional but recommended).
NOTE: In Method 16, a test run is composed of 16 individual analyses (injects) performed over a period of not less than 3 hours or more than 6 hours. For Method 16A to be consistent with Method 16, the following may be used to obtain a test run: (1) collect three 60-minute samples or (2) collect one 3-hour sample. (Three test runs constitute a test.)
8.4 Sample Recovery.
Disconnect the impingers. Quantitatively transfer the contents of the midget impingers of the Method 6 part of the train into a leak-free polyethylene bottle for shipment. Rinse the three midget impingers and the connecting tubes with water and add the washings to the same storage container. Mark the fluid level. Seal and identify the sample container.
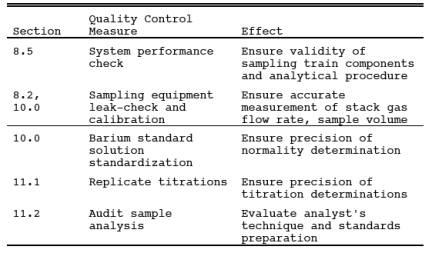
8.5 System Performance Check.
8.5.1 A system performance check is done (1) to validate the sampling train components and procedure (prior to testing; optional) and (2) to validate a test run (after a run). Perform a check in the field prior to testing consisting of a least two samples (optional), and perform an additional check after each 3 hour run or after three 1-hour samples (mandatory).
8.5.2 The checks involve sampling a known concentration of H2S and comparing the analyzed concentration with the known concentration. Mix the H2S recovery check gas (Section 7.1.4) and combustion gas in a dilution system such as that shown in Figure 16A-3. Adjust the flow rates to generate an H2S concentration in the range of the stack gas or within 20 percent of the applicable standard and an oxygen concentration greater than 1 percent at a total flow rate of at least 2.5 liters/min (5.3 ft3/hr). Use Equation 16A-3 to calculate the concentration of recovery gas generated. Calibrate the flow rate from both sources with a soap bubble flow meter so that the diluted concentration of H2S can be accurately calculated.
8.5.3 Collect 30-minute samples, and analyze in the same manner as the emission samples.
Collect the sample through the Probe of the sampling train using a manifold or some other suitable device that will ensure extraction of a representative sample.
8.5.4 The recovery check must be performed in the field prior to replacing the SO2 scrubber and particulate filter and before the Probe is cleaned. Use Equation 16A-4 (see Section 12.5) to calculate the recovery efficiency. Report the recovery efficiency with the emission data; do not correct the emission data for the recovery efficiency. A sample recovery of 100 ± 20 percent must be obtained for the emission data to be valid. However, if the recovery efficiency is not in the 100 ± 20 percent range but the results do not affect the compliance or noncompliance status of the affected facility, the Administrator may decide to accept the results of the compliance test.
9.0 Quality Control.
10.0 Calibration.
Same as Method 6, Section 10.0.
11.0 Analytical Procedure.
11.1 Sample Loss Check and Sample Analysis.
Same as Method 6, Sections 11.1 and 11.2, respectively, with the following exception: for 1-hour sampling, take a 40-ml aliquot, add 160 ml of 100 percent isopropanol and four drops of thorin.
11.2 Audit Sample Analysis.
Same as Method 6, Section 11.3.
12.0 Data Analysis and Calculations.
In the calculations, at least one extra decimal figure should be retained beyond that of the acquired data. Figures should be rounded off after final calculations.
12.1 Nomenclature.
CTRS = Concentration of TRS as SO>2, dry basis corrected to standard conditions, ppmv.
CRG(act) = Actual concentration of recovery check gas (after dilution), ppm.
CRG(m) = Measured concentration of recovery check gas generated, ppm.
CH2S= Verified concentration of H2S recovery gas.
N = Normality of barium perchlorate titrant, milliequivalents/ml.
Pbar = Barometric pressure at exit orifice of the console meter, mm Hg (in. Hg).
Pstd = Standard absolute pressure, 760 mm Hg (29.92 in. Hg).
QH2S= Calibrated flow rate of H2S recovery gas, liters/min.
QCG = Calibrated flow rate of combustion gas, liters/min.
R = Recovery efficiency for the system performance check, percent.
Tm = Average console meter absolute temperature, K (R).
Tstd = Standard absolute temperature, 293 K, (528R).
Va = Volume of sample aliquot titrated, ml.
Vm = Dry gas volume as measured by the console meter, liters (dcf).
Vm(std) = Dry gas volume measured by the console meter, corrected to standard conditions, liters (dscf).
Vsoln = Total volume of solution in which the sulfur dioxide sample is contained, 100 ml.
Vt = Volume of barium perchlorate titrant used for the sample, ml (average of replicate titrations).
Vtb = Volume of barium perchlorate titrant used for the blank, ml.
Y = console meter calibration factor.
32.03 = Equivalent weight of sulfur dioxide, mg/meq.
12.2 Dry Sample Gas Volume, Corrected to Standard Conditions.

where:
K1 = 0.3855 K/mm Hg for metric units,
= 17.65 R/in. Hg for English units.
12.3 Concentration of TRS as ppm SO2.
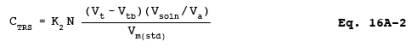
where:

12.4 Concentration of Recovery Gas Generated in the System Performance Check.

12.5 Recovery Efficiency for the System Performance Check.

13.0 Method Performance.
13.1 Analytical Range.
The lower detectable limit is 0.1 ppmv SO2 when sampling at 2 liters/min (4.2 ft3/hr) for 3 hours or 0.3 ppmv when sampling at 2 liters/min (4.2 ft3/hr) for 1 hour. The upper concentration limit of the method exceeds the TRS levels generally encountered at kraft pulp mills.
13.2 Precision.
Relative standard deviations of 2.0 and 2.6 percent were obtained when sampling a recovery boiler for 1 and 3 hours, respectively.
13.3 Bias.
13.3.1 No bias was found in Method 16A relative to Method 16 in a separate study at a recovery boiler. 13.3.2 Comparison of Method 16A with Method 16 at a lime kiln indicated that there was no bias in Method 16A. However, instability of the source emissions adversely affected the comparison. The precision of Method 16A at the lime kiln was similar to that obtained at the recovery boiler (Section 13.2.1).
13.3.3 Relative standard deviations of 2.7 and 7.7 percent have been obtained for system performance checks.
14.0 Pollution Prevention. [Reserved]
15.0 Waste Management. [Reserved]
16.0 Alternative Procedures.
As an alternative to the procedures specified in Section 7.1.4, the following procedure may be used to verify the H2S concentration of the recovery check gas.
16.1 Summary.
The H2S is collected from the calibration gas cylinder and is absorbed in zinc acetate solution to form zinc sulfide. The latter compound is then measured iodometrically.
16.2 Range.
The procedure has been examined in the range of 5 to 1500 ppmv.
16.3 Interferences.
There are no known interferences to this procedure when used to analyze cylinder gases containing H2S in nitrogen.
16.4 Precision and Bias.
Laboratory tests have shown a relative standard deviation of less than 3 percent. The procedure showed no bias when compared to a gas chromatographic method that used gravimetrically certified permeation tubes for calibration.
16.5 equipment and supplies.
16.5.1 Sampling Apparatus.
The sampling train is shown in Figure 16A-4. Its component parts are discussed in Sections 16.5.1.1 through 16.5.2.
16.5.1.1 Sampling Line.
Teflon tubing (1/4-in.) to connect the cylinder regulator to the sampling valve.
16.5.1.2 Needle Valve.
Stainless steel or Teflon needle valve to control the flow rate of gases to the impingers.
16.5.1.3 impingers.
Three impingers of approximately 100-ml capacity, constructed to permit the addition of reagents through the gas inlet stem. The impingers shall be connected in series with leak-free glass or Teflon connectors. The impinger bottoms have a standard 24/25 ground-glass fitting. The stems are from standard 6.4-mm (1/4-in.) ball joint midget impingers, custom lengthened by about 1 in. When fitted together, the stem end should be approximately 1.27 cm (1/2 in.) from the bottom (Southern Scientific, Inc., Micanopy, Florida: Set Number S6962-048). The third in-line impinger acts as a drop-out bottle.
16.5.1.4 Drying Tube, Rate meter, and barometer.
Same as Method 11, Sections 6.1.5, 6.1.8, and 6.1.10, respectively.
16.5.1.5 Cylinder Gas Regulator.
Stainless steel, to reduce the pressure of the gas stream entering the Teflon sampling line to a safe level.
16.5.1.6 Soap Bubble meter.
Calibrated for 100 and 500 ml, or two separate bubble meters.
16.5.1.7 Critical Orifice.
For volume and rate measurements. The critical orifice may be fabricated according to Section 16.7.3 and must be calibrated as specified in Section 16.12.4.
16.5.1.8 Graduated Cylinder.
50-ml size.
16.5.1.9 Volumetric Flask.
1-liter size.
16.5.1.10 Volumetric Pipette.
15-ml size.
16.5.1.11 Vacuum Gauge.
Minimum 20 in. Hg capacity.
16.5.1.12 Stopwatch.
16.5.2 Sample Recovery and Analysis.
16.5.2.1 Erlenmeyer Flasks.
125- and 250-ml sizes.
16.5.2.2 Pipettes.
2-, 10-, 20-, and 100-ml volumetric.
16.5.2.3 Burette.
50-ml size.
16.5.2.4 Volumetric Flask.
1-liter size.
16.5.2.5 Graduated Cylinder. 50-ml size.
16.5.2.6 Wash Bottle.
16.5.2.7 Stirring Plate and Bars.
16.6 Reagents and Standards.
Unless otherwise indicated, all reagents must conform to the specifications established by the Committee on Analytical Reagents of the American Chemical Society, where such specifications are available. Otherwise, use the best available grade.
16.6.1 Water.
Same as Method 11, Section 7.1.3.
16.6.2 Zinc Acetate Absorbing Solution.
Dissolve 20 g zinc acetate in water, and dilute to 1 liter.
16.6.3 Potassium Bi-iodate [KH(IO3)2] Solution, Standard 0.100 N.
Dissolve 3.249 g anhydrous KH(IO3)>2 in water, and dilute to 1 liter.
16.6.4 Sodium Thiosulfate (Na2S2O3) Solution, Standard 0.1 N.
Same as Method 11, Section 7.3.2. Standardize according to Section 16.12.2.
16.6.5 Na2S2O3 Solution, Standard 0.01 N. Pipette 100.0 ml of 0.1 N Na2S2O3 solution into a 1-liter volumetric flask, and dilute to the mark with water.
16.6.6 Iodine Solution, 0.1 N. Same as Method 11, Section 7.2.3.
16.6.7 Standard Iodine Solution, 0.01 N. Same as in Method 11, Section 7.2.4. Standardize according to Section 16.12.3.
16.6.8 Hydrochloric Acid (HCl) Solution, 10 Percent by Weight. Add 230 ml concentrated HCl (specific gravity 1.19) to 770 ml water.
16.6.9 Starch Indicator Solution. To 5 g starch (potato, arrowroot, or soluble), add a little cold water, and grind in a mortar to a thin paste. Pour into 1 liter of boiling water, stir, and let settle overnight. Use the clear supernatant. Preserve with 1.25 g salicylic acid, 4 g zinc chloride, or a combination of 4 g sodium propionate and 2 g sodium azide per liter of starch solution. Some commercial starch substitutes are satisfactory.
16.7 Pre-test Procedures.
16.7.1 Selection of Gas Sample Volumes. This procedure has been validated for estimating the volume of cylinder gas sample needed when the H2S concentration is in the range of 5 to 1500 ppmv. The sample volume ranges were selected in order to ensure a 35 to 60 percent consumption of the 20 ml of 0.01 N iodine (thus ensuring a 0.01 N Na2S2O3 titer of approximately 7 to 12 ml). The sample volumes for various H2S concentrations can be estimated by dividing the approximate ppm-liters desired for a given concentration range by the H2S concentration stated by the manufacturer. For example, for analyzing a cylinder gas containing approximately 10 ppmv H2S, the optimum sample volume is 65 liters (650 ppm-liters/10 ppmv). For analyzing a cylinder gas containing approximately 1000 ppmv H2S, the optimum sample volume is 1 liter (1000 ppm-liters/1000 ppmv).

16.7.2 Critical Orifice flow Rate Selection. The following table shows the ranges of sample flow rates that are desirable in order to ensure capture of H2S in the impinger solution. Slight deviations from these ranges will not have an impact on measured concentrations.
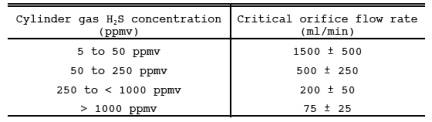
16.7.3 Critical Orifice Fabrication. Critical orifice of desired flow rates may be fabricated by selecting an orifice tube of desired length and connecting 1/16-in. x 1/4-in. (0.16 cm x 0.64 cm) reducing fittings to both ends. The inside diameters and lengths of orifice tubes needed to obtain specific flow rates are shown below.
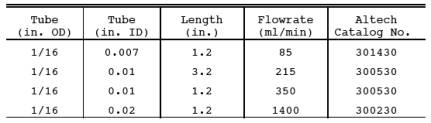
16.7.4 Determination of Critical Orifice Approximate flow Rate. Connect the critical orifice to the sampling system as shown in Figure 16A-4 but without the H2S cylinder. Connect a rotameter in the line to the first impinger. Turn on the pump, and adjust the valve to give a reading of about half atmospheric pressure. Observe the rotameter reading. Slowly increase the vacuum until a Approximate sampling time ’ Optimum volume Critical orifice flow rate stable flow rate is reached, and record this as the critical vacuum. The measured flow rate indicates the expected critical flow rate of the orifice. If this flow rate is in the range shown in Section 16.7.2, proceed with the critical orifice calibration according to Section 16.12.4.
16.7.5 Determination of Approximate Sampling Time. Determine the approximate sampling time for a cylinder of known concentration. Use the optimum sample volume obtained in Section 16.7.1.
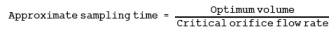
16.8 Sample Collection.
16.8.1 Connect the Teflon tubing, Teflon tee, and rotameter to the flow control needle valve as shown in Figure 16A-4. Vent the rotameter to an exhaust hood. Plug the open end of the tee. Five to 10 minutes prior to sampling, open the cylinder valve while keeping the flow control needle valve closed. Adjust the delivery pressure to 20 psi. Open the needle valve slowly until the rotameter shows a flow rate approximately 50 to 100 ml above the flow rate of the critical orifice being used in the system.
16.8.2 Place 50 ml of zinc acetate solution in two of the impingers, connect them and the empty third impinger (dropout bottle) and the rest of the equipment as shown in Figure 16A-4. Make sure the ground-glass fittings are tight. The impingers can be easily stabilized by using a small cardboard box in which three holes have been cut, to act as a holder. Connect the Teflon sample line to the first impinger. Cover the impingers with a dark cloth or piece of plastic to protect the absorbing solution from light during sampling.
16.8.3 Record the temperature and barometric pressure. Note the gas flow rate through the rotameter. Open the closed end of the tee. Connect the sampling tube to the tee, ensuring a tight connection. Start the sampling pump and stopwatch simultaneously. Note the decrease in flow rate through the excess flow rotameter. This decrease should equal the known flow rate of the critical orifice being used. Continue sampling for the period determined in Section 16.7.5.
16.8.4 When sampling is complete, turn off the pump and stopwatch. Disconnect the sampling line from the tee and plug it. Close the needle valve followed by the cylinder valve. Record the sampling time.
16.9 Blank Analysis.
While the sample is being collected, run a blank as follows: To a 250-ml Erlenmeyer flask, add 100 ml of zinc acetate solution, 20.0 ml of 0.01 N iodine solution, and 2 ml HCl solution. Titrate, while stirring, with 0.01 N Na2S2O3 until the solution is light yellow. Add starch, and continue titrating until the blue color disappears. Analyze a blank with each sample, as the blank titer has been observed to change over the course of a day.
NOTE: Iodine titration of zinc acetate solutions is difficult to perform because the solution turns slightly white in color near the end point, and the disappearance of the blue color is hard to recognize. In addition, a blue color may reappear in the solution about 30 to 45 seconds after the titration endpoint is reached. This should not be taken to mean the original endpoint was in error. It is recommended that persons conducting this test perform several titrations to be able to correctly identify the endpoint. The importance of this should be recognized because the results of this analytical procedure are extremely sensitive to errors in titration.
16.10 Sample Analysis.
Sample treatment is similar to the blank treatment. Before detaching the stems from the bottoms of the impingers, add 20.0 ml of 0.01 N iodine solution through the stems of the impingers holding the zinc acetate solution, dividing it between the two (add about 15 ml to the first impinger and the rest to the second). Add 2 ml HCl solution through the stems, dividing it as with the iodine. Disconnect the sampling line, and store the impingers for 30 minutes. At the end of 30 minutes, rinse the impinger stems into the impinger bottoms. Titrate the impinger contents with 0.01 N Na2S2O3. Do not transfer the contents of the impinger to a flask because this may result in a loss of iodine and cause a positive bias.
16.11 Post-test Orifice calibration.
Conduct a post-test critical orifice calibration run using the calibration procedures outlined in Section 16.12.4. If the Qstd obtained before and after the test differs by more than 5 percent, void the sample; if not, proceed to perform the calculations.
16.12 calibrations and Standardizations.
16.12.1 Rotameter and barometer. Same as Method 11, Sections 10.1.3 and 10.1.4.
16.12.2 Na2S2O3 Solution, 0.1 N. Standardize the 0.1 N Na2S2O3 solution as follows: To 80 ml water, stirring constantly, add 1 ml concentrated H2SO4, 10.0 ml of 0.100 N KH(IO3)2 and 1 g potassium iodide. Titrate immediately with 0.1 N Na2S2O3 until the solution is light yellow. Add 3 ml starch solution, and titrate until the blue color just disappears. Repeat the titration until replicate analyses agree within 0.05 ml. Take the average volume of Na2S2O3consumed to calculate the normality to three decimal figures using Equation 16A-5.
16.12.3 Iodine Solution, 0.01 N. Standardize the 0.01 N iodine solution as follows: Pipet 20.0 ml of 0.01 N iodine solution into a 125-ml Erlenmeyer flask. Titrate with standard 0.01 N Na2S2O3 solution until the solution is light yellow. Add 3 ml starch solution, and continue titrating until the blue color just disappears. If the normality of the iodine tested is not 0.010, add a few ml of 0.1 N iodine solution if it is low, or a few ml of water if it is high, and standardize again. Repeat the titration until replicate values agree within 0.05 ml. Take the average volume to calculate the normality to three decimal figures using Equation 16A-6.
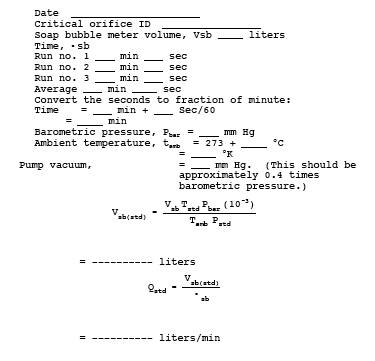
16.12.4 Critical Orifice. Calibrate the critical orifice using the sampling train shown in Figure 16A-4 but without the H2S cylinder and vent rotameter. Connect the soap bubble meter to the Teflon line that is connected to the first impinger. Turn on the pump, and adjust the needle valve until the vacuum is higher than the critical vacuum determined in Section 16.7.4. Record the time required for gas flow to equal the soap bubble meter volume (use the 100-ml soap bubble meter for gas flow rates below 100 ml/min, otherwise use the 500-ml soap bubble meter). Make three runs, and record the data listed in Table 16A-1. Use these data to calculate the volumetric flow rate of the orifice.
16.13 Calculations.
16.13.1 Nomenclature.
Bwa = Fraction of water vapor in ambient air during orifice calibration.
CH2S = H2S concentration in cylinder gas, ppmv.

Ma = Molecular weight of ambient air saturated at impinger temperature, g/g-mole.
Ms = Molecular weight of sample gas (nitrogen) saturated at impinger temperature, g/g-mole.
NOTE: (For tests carried out in a laboratory where the impinger temperature is 25 C, Ma = 28.5 g/g-mole and Ms= 27.7 g/g-mole.)
NI = Normality of standard iodine solution (0.01 N), g-eq/liter.
NT = Normality of standard Na2S2O3 solution (0.01 N), g-eq/liter.
Pbar = Barometric pressure, mm Hg.
Pstd = Standard absolute pressure, 760 mm Hg.
Qstd = Average volumetric flow rate through critical orifice, liters/min.
Tamb = Absolute ambient temperature, K.
Tstd = Standard absolute temperature, 293 K.
Os = Sampling time, min.
Osb = Time for soap bubble meter flow rate measurement, min.
Vm(std) = Sample gas volume measured by the critical orifice, corrected to standard conditions, liters.
Vsb = Volume of gas as measured by the soap bubble meter, ml.
Vsb(std)= Volume of gas as measured by the soap bubble meter, corrected to standard conditions, liters.
VI = Volume of standard iodine solution (0.01 N) used, ml.
VT = Volume of standard Na2S2O3 solution (0.01 N) used, ml.
VTB = Volume of standard Na2S2O3solution (0.01 N) used for the blank, ml.
16.13.2 Normality of Standard Na2S2O3 Solution (0.1 N).

16.13.3 Normality of Standard Iodine Solution (0.01 N).

16.13.4 Sample Gas Volume.

16.13.5 Concentration of H2S in the Gas Cylinder.

17.0 References.
1. American Public Health Association, American Water Works Association, and Water Pollution Control Federation. Standard Methods for the Examination of Water and Wastewater. Washington, DC. American Public Health Association. 1975. pp. 316-317.
2. American Society for Testing and Materials. Annual Book of ASTM Standards. Part 31: Water, Atmospheric Analysis. Philadelphia, PA. 1974. pp. 40-42.
3. Blosser, R.O. A Study of TRS Measurement Methods. National Council of the Paper Industry for Air and Stream Improvement, Inc., New York, NY. Technical Bulletin No. 434. May 1984. 14 pp.
4. Blosser, R.O., H.S. Oglesby, and A.K. Jain. A Study of Alternate SO2 Scrubber Designs Used for TRS Monitoring. A Special Report by the National Council of the Paper Industry for Air and Stream Improvement, Inc., New York, NY. July 1977.
5. Curtis, F., and G.D. McAlister. Development and Evaluation of an Oxidation/Method 6 TRS Emission Sampling Procedure. Emission Measurement Branch, Emission Standards and Engineering Division, U.S. Environmental Protection Agency, Research Triangle Park, NC 27711. February 1980.
6. Gellman, I. A Laboratory and Field Study of Reduced Sulfur Sampling and Monitoring Systems. National Council of the Paper Industry for Air and Stream Improvement, Inc., New York, NY. Atmospheric Quality Improvement Technical Bulletin No. 81. October 1975.
7. Margeson, J.H., J.E. Knoll, and M.R. Midgett. A Manual Method for TRS Determination. Source Branch, Quality Assurance Division, U.S. Environmental Protection Agency, Research Triangle Park, NC 27711.
8. National Council of the Paper Industry for Air and Stream Improvement. An Investigation of H2S and SO2. calibration Cylinder Gas Stability and Their Standardization Using Wet Chemical Techniques. Special Report 76-06. New York, NY. August 1976.
9. National Council of the Paper Industry for Air and Stream Improvement. Wet Chemical Method for Determining the H2S Concentration of calibration Cylinder Gases. Technical Bulletin Number 450. New York, NY. January 1985. 23 pp.
10. National Council of the Paper Industry for Air and Stream Improvement. Modified Wet Chemical Method for Determining the H2S Concentration of calibration Cylinder Gases. Draft Report. New York, NY. March 1987. 29 pp.
18.0 Tables, Diagrams, flowcharts, and Validation Data.
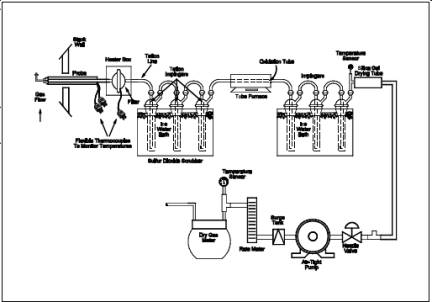
Figure 16A-2. Angled Sampling Probe.
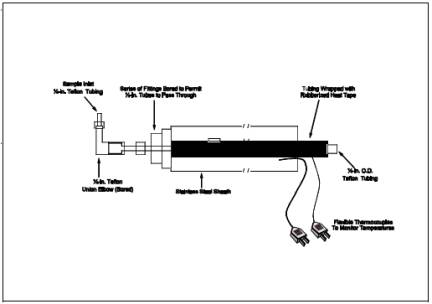
Figure 16A-3. Recovery Gas Dilution System.
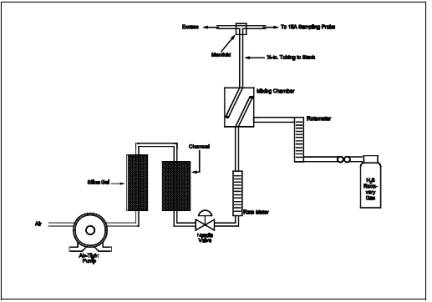
Figure 16A-4. Recovery Check Gas Sampling Train.
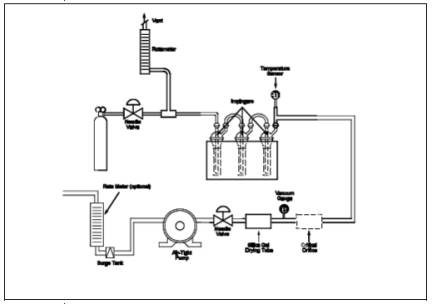
Table 16A-1. Critical Orifice calibration Data.
- Analytical
- Ion Chromatography
- Gas Chromatography
- Gravimetrics
- Ash Resistivity
- Inks/Coatings
- Scrubber Stoichiometry
- Titrations
- Mercury Sorbent Trap
- Engineering
- Express Products
- Rental Instruments
- MET80 Mercury Monitor
- Continuous Emission Monitors
- Gas Sampling Equipment
- Mobile Power Supply
Our Resources
- Technical Resources

 Express
Express FTIR
FTIR Mercury
Mercury Emission Sampling Equipment
Emission Sampling Equipment Instrument Rental
Instrument Rental