EPA Methods List with Links
US EPA Method 7b - Determination Of Nitrogen Oxide Emissions From Stationary Sources (Ultraviolet Spectrophotometric Method)
NOTE: This method does not include all of the specifications (e.g., equipment and supplies) and procedures (e.g., sampling and analytical) essential to its performance. Some material is incorporated by reference from other methods in this part. Therefore, to obtain reliable results, persons using this method should have a thorough knowledge of at least the following additional test methods: Method 1, Method 5, and Method 7.
1.0 Scope and Application.
1.1 Analytes.
| Analyte | CAS No. | Sensitivity |
| Nitrogen oxides (NOx), as NO2, including: Nitric oxide (NO) Nitrogen dioxide (NO2) |
10102-43-9 10102-44-0 |
30-786 ppmv |
1.2 Applicability.
This method is applicable for the determination of NOx emissions from nitric acid plants.
1.3 Data Quality Objectives.
Adherence to the requirements of this method will enhance the quality of the data obtained from air pollutant sampling methods.
2.0 Summary of Method.
2.1 A grab sample is collected in an evacuated flask containing a dilute sulfuric acid-hydrogen peroxide absorbing solution; the NOx, excluding nitrous oxide (N2O), are measured by ultraviolet spectrophotometry.
3.0 Definition. [Reserved]
4.0 Interferences. [Reserved]
5.0 Safety.
5.1 This method may involve hazardous materials, operations, and equipment. This test method may not address all of the safety problems associated with its use. It is the responsibility of the user of this test method to establish appropriate safety and health practices and to determine the applicability of regulatory limitations prior to performing this test method.
5.2 Corrosive reagents.
The following reagents are hazardous. Personal protective equipment and safe procedures are useful in preventing chemical splashes. If contact occurs, immediately flush with copious amounts of water at least 15 minutes. Remove clothing under shower and decontaminate. Treat residual chemical burn as thermal burn.
5.2.1 Hydrogen Peroxide (H2O2).
Irritating to eyes, skin, nose, and lungs.
5.2.2 Sodium Hydroxide (NaOH).
Causes severe damage to eyes and skin. Inhalation causes irritation to nose, throat, and lungs. Reacts exothermically with limited amounts of water.
5.2.3 Sulfuric Acid (H2SO4).
Rapidly destructive to body tissue. Will cause third degree burns. Eye damage may result in blindness. Inhalation may be fatal from spasm of the larynx, usually within 30 minutes. May cause lung tissue damage with edema. 3 mg/m3 will cause lung damage in uninitiated. 1 mg/m3 for 8 hours will cause lung damage or, in higher concentrations, death. Provide ventilation to limit inhalation. Reacts violently with metals and organics.
6.0 Equipment and Supplies.
6.1 Sample Collection.
Same as Method 7, Section 6.1.
6.2 Sample Recovery.
The following items are required for sample recovery:
6.2.1 Wash Bottle.
Polyethylene or glass.
6.2.2 Volumetric Flasks.
100-ml (one for each sample).
6.3 Analysis.
The following items are required for analysis:
6.3.1 Volumetric Pipettes.
5-, 10-, 15-, and 20-ml to make standards and sample dilutions.
6.3.2 Volumetric Flasks.
1000- and 100-ml for preparing standards and dilution of samples.
6.3.3 Spectrophotometer.
To measure ultraviolet absorbance at 210 nm.
6.3.4 Analytical Balance.
To measure to within 0.1 mg.
7.0 Reagents and Standards.
NOTE: Unless otherwise indicated, all reagents are to conform to the specifications established by the Committee on Analytical Reagents of the American Chemical Society, where such specifications are available. Otherwise, use the best available grade.
7.1 Sample Collection.
Same as Method 7, Section 7.1. It is important that the amount of hydrogen peroxide in the absorbing solution not be increased. Higher concentrations of peroxide may interfere with sample analysis.
7.2 Sample Recovery.
Same as Method 7, Section 7.2.
7.3 Analysis.
Same as Method 7, Sections 7.3.1, 7.3.3, and 7.3.4, with the addition of the following:
7.3.1 Working Standard KNO3 Solution.
Dilute 10 ml of the standard solution to 1000 ml with water. One milliliter of the working standard is equivalent to 10 μg NO2.
8.0 Sample Collection, Preservation, Storage, and Transport.
8.1 Sample Collection.
Same as Method 7, Section 8.1.
8.2 Sample Recovery.
8.2.1 Let the flask sit for a minimum of 16 hours, and then shake the contents for 2 minutes.
8.2.2 Connect the flask to a mercury filled U-tube manometer. Open the valve from the flask to the manometer, and record the flask temperature (Tf), the barometric pressure, and the difference between the mercury levels in the manometer. The absolute internal pressure in the flask (Pf) is the barometric pressure less the manometer reading.
8.2.3 Transfer the contents of the flask to a leak- free wash bottle. Rinse the flask three times with 10-ml portions of water, and add to the bottle. Mark the height of the liquid level so that the container can be checked for leakage after transport. Label the container to identify clearly its contents. Seal the container for shipping.
9.0 Quality Control.
| Section | Quality Control Measure | Effect |
| 10.1 | Spectrophometer calibration | Ensures linearity of spectrophotometer response to standards |
| 11.4 | Audit sample analysis | Evaluates analytical technique and preparation of standards |
10.0 Calibration and Standardizations.
Same as Method 7, Sections 10.2 through 10.5, with the addition of the following:
10.1 Determination of Spectrophotometer Standard Curve.
Add 0 ml, 5 ml, 10 ml, 15 ml, and 20 ml of the KNO3 working standard solution (1 ml = 10 μg NO2) to a series of five 100-ml volumetric flasks. To each flask, add 5 ml of absorbing solution. Dilute to the mark with water. The resulting solutions contain 0.0, 50, 100, 150, and 200 μg NO2, respectively. Measure the absorbance by ultraviolet spectrophotometry at 210 nm, using the blank as a zero reference. Prepare a standard curve plotting absorbance vs. μg NO2.
NOTE: If other than a 20-ml aliquot of sample is used for analysis, then the amount of absorbing solution in the blank and standards must be adjusted such that the same amount of absorbing solution is in the blank and standards as is in the aliquot of sample used.
10.1.1 Calculate the spectrophotometer calibration factor as follows:
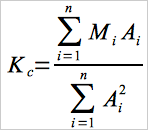 Eq. 7B-1
Eq. 7B-1
where:
| Mi | = | Mass of NO2 in standard i, μg. |
| Ai | = | Absorbance of NO2 standard i. |
| n | = | Total number of calibration standards. |
10.1.2 For the set of calibration standards specified here, Equation 7B-1 simplifies to the following:
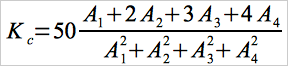 Eq. 7B-2
Eq. 7B-2
10.2 Spectrophotometer calibration Quality Control.
Multiply the absorbance value obtained for each standard by the Kc factor (reciprocal of the least squares slope) to determine the distance each calibration point lies from the theoretical calibration line. The difference between the calculated concentration values and the actual concentrations (i.e., 50, 100, 150, and 200 μg NO2) should be less than 7 percent for all standards.
11.0 Analytical Procedures.
11.1 Sample Loss Check.
Note the level of the liquid in the container, and confirm whether any sample was lost during shipment. Note this on the analytical data sheet. If a noticeable amount of leakage has occurred, either void the sample or use methods, subject to the approval of the Administrator, to correct the final results.
11.2 Sample Preparation.
Immediately prior to analysis, transfer the contents of the shipping container to a 100-ml volumetric flask, and rinse the container twice with 5-ml portions of water. Add the rinse water to the flask, and dilute to mark with water.
11.3 Sample Analysis.
Mix the contents of the flask thoroughly and pipette a 20 ml-aliquot of sample into a 100- ml volumetric flask. Dilute to the mark with water. Using the blank as zero reference, read the absorbance of the sample at 210 nm.
11.4 Audit Sample Analysis.
Same as Method 7, Section 11.4, except that a set of audit samples must be analyzed with each set of compliance samples or once per analysis day, or once per week when averaging continuous samples.
12.0 Data Analysis and Calculations.
Same as Method 7, Section 12.0, except replace Section 12.3 with the following:
12.1 Total μg NO2 Per Sample.
 Eq. 7B-3
Eq. 7B-3
where:
5 = 100/20, the aliquot factor.
NOTE: If other than a 20-ml aliquot is used for analysis, the factor 5 must be replaced by a corresponding factor.
13.0 Method Performance.
13.1 Range.
The analytical range of the method as outlined has been determined to be 57 to 1500 milligrams NOx (as NO2) per dry standard cubic meter, or 30 to 786 parts per million by volume (ppmv) NOx.
14.0 Pollution Prevention. [Reserved]
15.0 Waste Management. [Reserved]
16.0 References.
1. National Institute for Occupational Safety and Health. Recommendations for Occupational Exposure to Nitric Acid. In: Occupational Safety and Health Reporter. Washington, D.C. Bureau of National Affairs, Inc. 1976. p. 149.
2. Rennie, P.J., A.M. Sumner, and F.B. Basketter. Determination of Nitrate in Raw, Potable, and Waste Waters by Ultraviolet Spectrophotometry. Analyst. 104:837. September 1979.
17.0 Tables, Diagrams, flowcharts, and Validation Data. [Reserved]
- Analytical
- Ion Chromatography
- Gas Chromatography
- Gravimetrics
- Ash Resistivity
- Inks/Coatings
- Scrubber Stoichiometry
- Titrations
- Mercury Sorbent Trap
- Engineering
- Express Products
- Rental Instruments
- MET80 Mercury Monitor
- Continuous Emission Monitors
- Gas Sampling Equipment
- Mobile Power Supply
Our Resources
- Technical Resources

 Express
Express FTIR
FTIR Mercury
Mercury Emission Sampling Equipment
Emission Sampling Equipment Instrument Rental
Instrument Rental