EPA Methods List with Links
US EPA Method 318 - Extractive FTIR Method for the Measurement of Emissions from the Mineral Wool and Wool Fiberglass Industries
APPENDIX A TO PART 63--TEST METHOD
Content [ show/hide ].1.0 Scope and Application.
This method has been validated and approved for mineral wool and wool fiberglass sources. This method may not be applied to other source categories without validation and approval by the Administrator according to the procedures in Test Method 301, 40 CFR Part 63, Appendix A. For sources seeking to apply FTIR to other source categories, Test Method 320 (40 CFR Part 63, Appendix A) may be utilized.
1.1 Scope.
The analytes measured by this method and their CAS numbers are:
Carbon Monoxide 630-08-0
Carbonyl Sulfide 463-58-1
Formaldehyde 50-00-0
Methanol 1455-13-6
Phenol 108-95-2
1.2 Applicability.
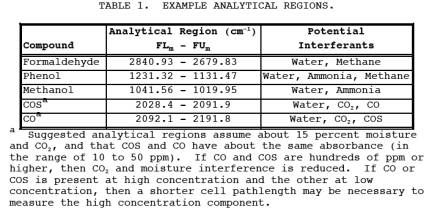
1.2.1 This method is applicable for the determination of formaldehyde, phenol, methanol, carbonyl sulfide (COS) and carbon monoxide (CO) concentrations in controlled and uncontrolled emissions from manufacturing processes using phenolic resins. The compounds are analyzed in the mid-infrared spectral region (about 400 to 4000 cm-1 or 25 to 2.5 Fm). Suggested analytical regions are given below (Table 1). Slight deviations from these recommended regions may be necessary due to variations in moisture content and ammonia concentration from source to source.
1.2.2 This method does not apply when: (a) polymerization of formaldehyde occurs, (b) moisture condenses in either the sampling system or the instrumentation, and (c) when moisture content of the gas stream is so high relative to the analyte concentrations that it causes severe spectral interference.
1.3 Method Range and Sensitivity.
1.3.1 The analytical range is a function of instrumental design and composition of the gas stream. Theoretical detection limits depend, in part, on (a) the absorption coefficient of the compound in the analytical frequency region, (b) the spectral resolution, (c) interferometer sampling time, (d) detector sensitivity and response, and (e) absorption path-length.
1.3.2 Practically, there is no upper limit to the range. The practical lower detection limit is usually higher than the theoretical value, and depends on (a) moisture content of the flue gas, (b) presence of interferants, and (c) losses in the sampling system. In general, a 22 meter path-length cell in a suitable sampling system can achieve practical detection limits of 1.5 ppm for three compounds (formaldehyde, phenol, and methanol) at moisture levels up to 15 percent by volume. Sources with uncontrolled emissions of CO and COS may require a 4 meter path-length cell due to high concentration levels. For these two compounds, make sure absorbance of highest concentration component is <1.0.
1.4 Data Quality Objectives.
1.4.1 In designing or configuring the system, the analyst first sets the data quality objectives, i.e., the desired lower detection limit (DLi) and the desired analytical uncertainty (AUi) for each compound. The instrumental parameters (factors b, c, d, and e in Section 1.3.1) are then chosen to meet these requirements, using Appendix D of the FTIR Protocol.
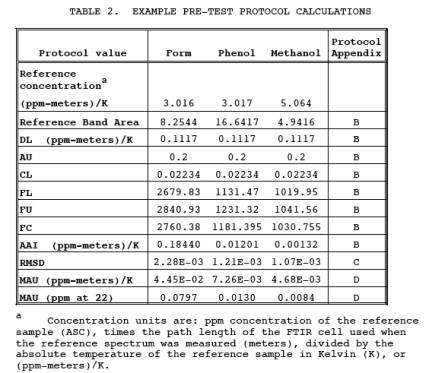
1.4.2 Data quality for each application is determined, in part, by measuring the RMS (Root Mean Square) noise level in each analytical spectral region (Appendix C of the FTIR Protocol). The RMS noise is defined as the RMSD (Root Mean Square Deviation) of the absorbance values in an analytical region from the mean absorbance value of the region. Appendix D of the FTIR Protocol defines the MAUim (minimum analyte uncertainty of the ith analyte in the mth analytical region). The MAU is the minimum analyte concentration for which the analytical uncertainty limit (AUi) can be maintained: if the measured analyte concentration is less than MAUi, then data quality is unacceptable. Table 2 gives some example DL and AU values along with calculated areas and MAU values using the protocol procedures.
2.0 Summary of Method.
2.1 Principle.
2.1.1 Molecules are composed of chemically bonded atoms, which are in constant motion. The atomic motions result in bond deformations (bond stretching and bond-angle bending). The number of fundamental (or independent) vibrational motions depends on the number of atoms (N) in the molecule. At typical testing temperatures, most molecules are in the ground-state vibrational state for most of their fundamental vibrational motions. A molecule can undergo a transition from its ground state (for a particular vibration) to the first excited state by absorbing a quantum of light at a frequency characteristic of the molecule and the molecular motion. Molecules also undergo rotational transitions by absorbing energies in the far-infrared or microwave spectral regions. Rotational transition absorbencies are superimposed on the vibrational absorbencies to give a characteristic shape to each rotational-vibrational absorbance "band."
2.1.2 Most molecules exhibit more than one absorbance band in several frequency regions to produce an infrared spectrum (a characteristic pattern of bands or a "fingerprint") that is unique to each molecule. The infrared spectrum of a molecule depends on its structure (bond lengths, bond angles, bond strengths, and atomic masses). Even small differences in structure can produce significantly different spectra.
2.1.3 Spectral band intensities vary with the concentration of the absorbing compound. Within constraints, the relationship between absorbance and sample concentration is linear. Sample spectra are compared to reference spectra to determine the species and their concentrations.
2.2 Sampling and Analysis.
2.2.1 flue gas is continuously extracted from the source, and the gas or a portion of the gas is conveyed to the FTIR gas cell, where a spectrum of the flue gas is recorded. Absorbance band intensities are related to sample concentrations by Beer's Law.

where:

2.2.2 After identifying a compound from the infrared spectrum, its concentration is determined by comparing band intensities in the sample spectrum to band intensities in "reference spectra" of the formaldehyde, phenol, methanol, COS and CO. These reference spectra are available in a permanent soft copy from the EPA spectral library on the EMTIC bulletin board. The source may also prepare reference spectra according to Section 4.5 of the FTIR Protocol. (Note: Reference spectra not prepared according to the FTIR Protocol are not acceptable for use in this test method. Documentation detailing the FTIR Protocol steps used in preparing any non-EPA reference spectra shall be included in each test report submitted by the source.)
2.3 Operator Requirements.
The analyst must have some knowledge of source sampling and of infrared spectral patterns to operate the sampling system and to choose a suitable instrument configuration. The analyst should also understand FTIR instrument operation well enough to choose an instrument configuration consistent with the data quality objectives.
3.0 Definitions.
See Appendix A of the FTIR Protocol.
4.0 Interferences.
4.1 Analytical (or Spectral) Interferences.
Water vapor. High concentrations of ammonia (hundreds of ppm) may interfere with the analysis of low concentrations of methanol (1 to 5 ppm). For CO, carbon dioxide and water may be interferants. In cases where COS levels are low relative to CO levels, CO and water may be interferants.
4.2 Sampling System Interferences.
Water, if it condenses, and ammonia, which reacts with formaldehyde.
5.0 Safety.
5.1 Formaldehyde is a suspected carcinogen; therefore, exposure to this compound must be limited. Proper monitoring and safety precautions must be practiced in any atmosphere with potentially high concentrations of CO.
5.2 This method may involve sampling at locations having high positive or negative pressures, high temperatures, elevated heights, high concentrations of hazardous or toxic pollutants, or other diverse sampling conditions. It is the responsibility of the tester(s) to ensure proper safety and health practices, and to determine the applicability of regulatory limitations before performing this test method.
6.0 Equipment and Supplies.
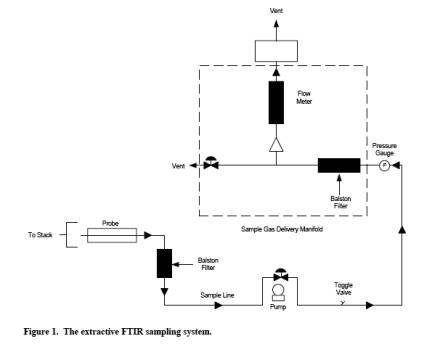
The equipment and supplies are based on the schematic of a sampling train shown in Figure 1. Either the evacuated or purged sampling technique may be used with this sampling train. Alternatives may be used, provided that the data quality objectives of this method are met.
6.1 Sampling Probe. glass, stainless steel, or other appropriate material of sufficient length and physical integrity to sustain heating, prevent adsorption of analytes, and to reach gas sampling point.
6.2 Particulate filters. A glass wool plug (optional) inserted at the Probe tip (for large particulate removal) and a filter rated at 1-micron (e.g., Balston™) for fine particulate removal, placed immediately after the heated Probe.
6.3 Sampling Line/Heating System. Heated (maintained at 250 ± 25 degrees F) stainless steel, TeflonTM, or other inert material that does not adsorb the analytes, to transport the sample to analytical system.
6.4 Stainless Steel tubing. Type 316, e.g., 3/8 in. diameter, and appropriate length for heated connections.
6.5 Gas Regulators. Appropriate for individual gas cylinders.
6.6 TeflonTM tubing. Diameter (e.g., 3/8 in.) and length suitable to connect cylinder regulators.
6.7 sample pump>. A leak-free pump (e.g., KNF™), with by-pass valve, capable of pulling sample through entire sampling system at a rate of about 10 to 20 L/min. If placed before the analytical system, heat the pump and use a pump fabricated from materials non-reactive to the target pollutants. If the pump is located after the instrument, systematically record the sample pressure in the gas cell.
6.8 Gas Sample Manifold. A heated manifold that diverts part of the sample stream to the analyzer, and the rest to the by-pass discharge vent or other analytical instrumentation.
6.9 Rotameter. A calibrated 0 to 20 L/min range rotameter.
6.10 FTIR Analytical System. Spectrometer and detector, capable of measuring formaldehyde, phenol, methanol, COS and CO to the predetermined minimum detectable level. The system shall include a personal computer with compatible software that provides real-time updates of the spectral profile during sample collection and spectral collection.
6.11 FTIR Cell pump. Required for the evacuated sampling technique, capable of evacuating the FTIR cell volume within 2 minutes. The FTIR cell pump should allow the operator to obtain at least 8 sample spectra in 1 hour.
6.12 Absolute Pressure Gauge. Heatable and capable of measuring pressure from 0 to 1000 mmHg to within ±2.5 mmHg (e.g., Baratron™).
6.13 temperature Gauge. Capable of measuring the cell temperature to within ±2C.
7.0 Reagents and Standards.
7.1 Ethylene (calibration Transfer Standard). Obtain NIST traceable (or Protocol) cylinder gas.
7.2 Nitrogen. Ultra high purity (UHP) grade.
7.3 Reference Spectra. Obtain reference spectra for the target pollutants at concentrations that bracket (in ppm-meter/K) the emission source levels. Also, obtain reference spectra for SF6 and ethylene. Suitable concentrations are 0.0112 to 0.112 (ppm-meter)/K for SF6 and 5.61 (ppm-meter)/K or less for ethylene. The reference spectra shall meet the criteria for acceptance outlined in Section 2.2.2. The optical density (ppm-meters/K) of the reference spectrum must match the optical density of the sample spectrum within (less than) 25 percent.
8.0 Sample Collection, Preservation, and Storage.
Sampling should be performed in the following sequence: Collect background, collect CTS spectrum, collect samples, collect post-test CTS spectrum, verify that two copies of all data were stored on separate computer media.
8.1 Pretest Preparations and Evaluations.
Using the procedure in Section 4.0 of the FTIR Protocol, determine the optimum sampling system configuration for sampling the target pollutants. Table 2 gives some example values for AU, DL, and MAU. Based on a study (Reference 1), an FTIR system using 1 cm-1 resolution, 22 meter path length, and a broad band MCT detector was suitable for meeting the requirements in Table 2. Other factors that must be determined are:
a. Test requirements: AUi, CMAXi, DLi, OFUi, and tAN for each.
b. Inteferants: See Table 1.
c. Sampling system: LS', Pmin, PS', TS', tSS, VSS; fractional error, MIL.
d. Analytical regions: 1 through Nm, FLm, FCm, and FUm, plus interferants, FFUm, FFLm, wave number range FNU to FNL. See Tables 1 and 2.
8.1.1 If necessary, sample and acquire an initial spectrum. Then determine the proper operational path-length of the instrument to obtain non-saturated absorbances of the target analytes.
8.1.2 Set up the sampling train as shown in Figure 1.
8.2 Sampling System Leak-check.
Leak-check from the Probe tip to pump outlet as follows: Connect a 0- to 250-mL/min rate meter (rotameter or bubble meter) to the outlet of the pump. Close off the inlet to the Probe, and note the leakage rate. The leakage rate shall be < 200 mL/min.
8.3 Analytical System Leak-check.
8.3.1 For the evacuated sample technique, close the valve to the FTIR cell, and evacuate the absorption cell to the minimum absolute pressure Pmin. Close the valve to the pump, and determine the change in pressure •Pv after 2 minutes.
8.3.2 For both the evacuated sample and purging techniques, pressurize the system to about 100 mmHg above atmospheric pressure. Isolate the pump and determine the change in pressure •Pp after 2 minutes.
8.3.3 Measure the barometric pressure, Pb in mmHg.
8.3.4 Determine the percent leak volume %VL for the signal integration time tSS and for •Pmax, i.e., the larger of •Pv or •Pp, as follows:

where:
50 = 100% divided by the leak-check time of 2 minutes.
8.3.5 Leak volumes in excess of 4 percent of the sample system volume VSS are unacceptable.
8.4 Background Spectrum.
Evacuate the gas cell to <5 mmHg, and fill with dry nitrogen gas to ambient pressure. Verify that no significant amounts of absorbing species (for example water vapor and CO2) are present. Collect a background spectrum, using a signal averaging period equal to or greater than the averaging period for the sample spectra. Assign a unique file name to the background spectrum. Store the spectra of the background interferogram and processed single-beam background spectrum on two separate computer media (one is used as the backup). If continuous sampling will be used during sample collection, collect the background spectrum with nitrogen gas flowing through the cell at the same pressure and temperature as will be used during sampling.
8.5 Pre-Test calibration Transfer Standard.
Evacuate the gas cell to <5 mmHg absolute pressure, and fill the FTIR cell to atmospheric pressure with the CTS gas. Or, purge the cell with 10 cell volumes of CTS gas. Record the spectrum. If continuous sampling will be used during sample collection, collect the CTS spectrum with CTS gas flowing through the cell at the same pressure and temperature as will be used during sampling.
8.6 Samples.
8.6.1 Evacuated Samples.
Evacuate the absorbance cell to <5 mmHg absolute pressure. Fill the cell with flue gas to ambient pressure and record the spectrum. Before taking the next sample, evacuate the cell until no further evidence of absorption exists. Repeat this procedure to collect at least 8 separate spectra (samples) in 1 hour.
8.6.2 Purge Sampling.
Purge the FTIR cell with 10 cell volumes of flue gas and at least for about 10 minutes. Discontinue the gas cell purge, isolate the cell, and record the sample spectrum and the pressure. Before taking the next sample, purge the cell with 10 cell volumes of flue gas.
8.6.3 Continuous Sampling.
Spectra can be collected continuously while the FTIR cell is being purged. The sample integration time, tss, the sample flow rate through the FTIR gas cell, and the total run time must be chosen so that the collected data consist of at least 10 spectra with each spectrum being of a separate cell volume of flue gas. More spectra can be collected over the run time and the total run time (and number of spectra) can be extended as well.
8.7 Sampling QA, Data Storage and Reporting.
8.7.1 Sample integration times should be sufficient to achieve the required signal-to-noise ratios. Obtain an absorbance spectrum by filling the cell with nitrogen. Measure the RMSD in each analytical region in this absorbance spectrum. Verify that the number of scans is sufficient to achieve the target MAU (Table 2).
8.7.2 Identify all sample spectra with unique file names.
8.7.3 Store on two separate computer media a copy of sample interferograms and processed spectra. The data shall be available to the Administrator on request for the length of time specified in the applicable regulation.
8.7.4 For each sample spectrum, document the sampling conditions, the sampling time (while the cell was being filled), the time the spectrum was recorded, the instrumental conditions (path length, temperature, pressure, resolution, integration time), and the spectral file name. Keep a hard copy of these data sheets.
8.8 Signal Transmittance.
While sampling, monitor the signal transmittance through the instrumental system. If signal transmittance (relative to the background) drops below 95 percent in any spectral region where the sample does not absorb infrared energy, obtain a new background spectrum.
8.9 Post-run CTS.
After each sampling run, record another CTS spectrum.
8.10 Post-test QA.
8.10.1 Inspect the sample spectra immediately after the run to verify that the gas matrix composition was close to the expected (assumed) gas matrix.
8.10.2 Verify that the sampling and instrumental parameters were appropriate for the conditions encountered. For example, if the moisture is much greater than anticipated, it will be necessary to use a shorter path length or dilute the sample.
8.10.3 Compare the pre- and post-run CTS spectra. They shall agree to within ±5 percent. See FTIR Protocol, Appendix E.
9.0 Quality Control.
Follow the quality assurance procedures in the method, including the analysis of pre- and post-run calibration transfer standards (Sections 8.5 and 8.9) and the post-test quality assurance procedures in Section 8.10.
10.0 Calibration and Standardization.
10.1 Signal-to-Noise Ratio (S/N).
The S/N shall be sufficient to meet the MAU in each analytical region.
10.2 Absorbance Path-length.
Verify the absorbance path length by comparing CTS spectra to reference spectra of the calibration gas(es). See FTIR Protocol, Appendix E.
10.3 Instrument Resolution.
Measure the line width of appropriate CTS band(s) and compare to reference CTS spectra to verify instrumental resolution.
10.4 Apodization Function.
Choose appropriate apodization function. Determine any appropriate mathematical transformations that are required to correct instrumental errors by measuring the CTS. Any mathematical transformations must be documented and reproducible.
10.5 FTIR Cell Volume.
Evacuate the cell to <5 mmHg. Measure the initial absolute temperature (Ti) and absolute pressure (Pi). Connect a wet test meter (or a calibrated console meter), and slowly draw room air into the cell. Measure the meter volume (Vm), meter absolute temperature (Tm), and meter absolute pressure (Pm), and the cell final absolute temperature (Tf) and absolute pressure (Pf). Calculate the FTIR cell volume VSS, including that of the connecting tubing, as follows:

As an alternative to the wet test meter/calibrated console meter procedure, measure the inside dimensions of the cell cylinder and calculate its volume.
11.0 Procedure.
Refer to Sections 4.6 - 4.11, Sections 5, 6, and 7, and the appendices of the FTIR Protocol.
12.0 Data Analysis and Calculations.
a. Data analysis is performed using appropriate reference spectra whose concentrations can be verified using CTS spectra. Various analytical programs are available to relate sample absorbance to a concentration standard. Calculated concentrations should be verified by analyzing spectral baselines after mathematically subtracting scaled reference spectra from the sample spectra. A full description of the data analysis and calculations may be found in the FTIR Protocol (Sections 4.0, 5.0, 6.0 and appendices).
b. Correct the calculated concentrations in sample spectra for differences in absorption path-length between the reference and sample spectra by:

where:

13.0 Reporting and Recordkeeping.
All interferograms used in determining source concentration shall be stored for the period of time required in the applicable regulation. The Administrator has the option of requesting the interferograms recorded during the test in electronic form as part of the test report.
14.0 Method Performance.
Refer to the FTIR Protocol.
15.0 Pollution Prevention. [Reserved]
16.0 Waste Management.
Laboratory standards prepared from the formaldehyde and phenol are handled according to the instructions in the materials safety data sheets (MSDS).
17.0 References.
(1) "Field Validation Test Using Fourier Transform Infrared (FTIR) Spectrometry To Measure Formaldehyde, Phenol and Methanol at a Wool Fiberglass Production Facility." Draft. U.S. Environmental Protection Agency Report, Entropy, Inc., EPA Contract No. 68D20163, Work Assignment I-32, December 1994 (docket item II-A-13).
(2) "Method 301 - Field Validation of Pollutant Measurement Methods from Various Waste Media," 40 CFR part 63, appendix A.
- Analytical
- Ion Chromatography
- Gas Chromatography
- Gravimetrics
- Ash Resistivity
- Inks/Coatings
- Scrubber Stoichiometry
- Titrations
- Mercury Sorbent Trap
- Engineering
- Express Products
- Rental Instruments
- MET80 Mercury Monitor
- Continuous Emission Monitors
- Gas Sampling Equipment
- Mobile Power Supply
Our Resources
- Technical Resources

 Express
Express FTIR
FTIR Mercury
Mercury Emission Sampling Equipment
Emission Sampling Equipment Instrument Rental
Instrument Rental